Leukapheresis Market Size, Share & Trends Analysis Report By Product type (Leukapheresis Devices, Centrifugal Devices, Membrane Separators, Leukapheresis Disposables), By Application (Research Application, Therapeutics Application), By End Use (BoldoCenters, Academic & Research Institutes, Pharmaceutical & Biotechnology Companies, Hospitals & Clinics) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Leukapheresis Market Overview
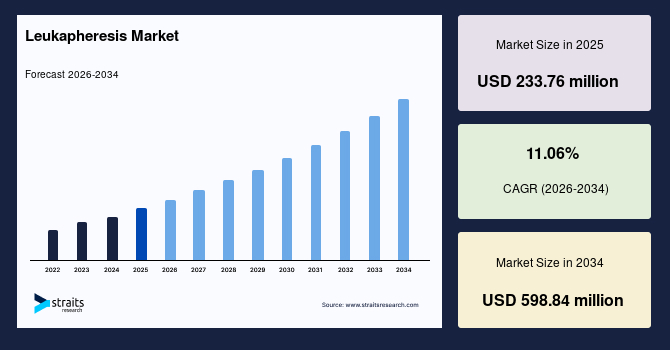
The global leukapheresis market size is valued at USD 233.76 million in 2025 and is estimated to reach USD 598.84 million by 2034, growing at a CAGR of 11.06% during the forecast period. The market growth is accelerated by the rapid increase in CAR-T therapy clinical trials worldwide, as each trial requires leukapheresis as the initial step for collecting patient-derived white blood cells, driving sustained demand across research and therapeutic settings.
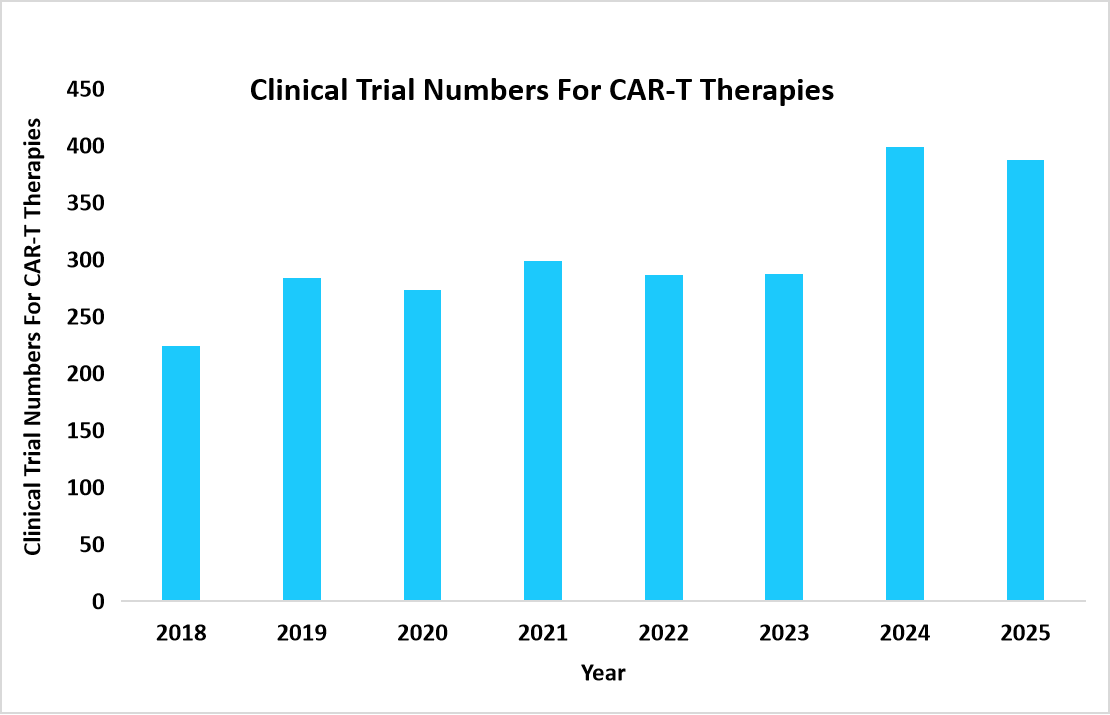
Source: GlobalData Pharmaceutical Intelligence Center, Clinical Trials database
Key Market Trends & Insights
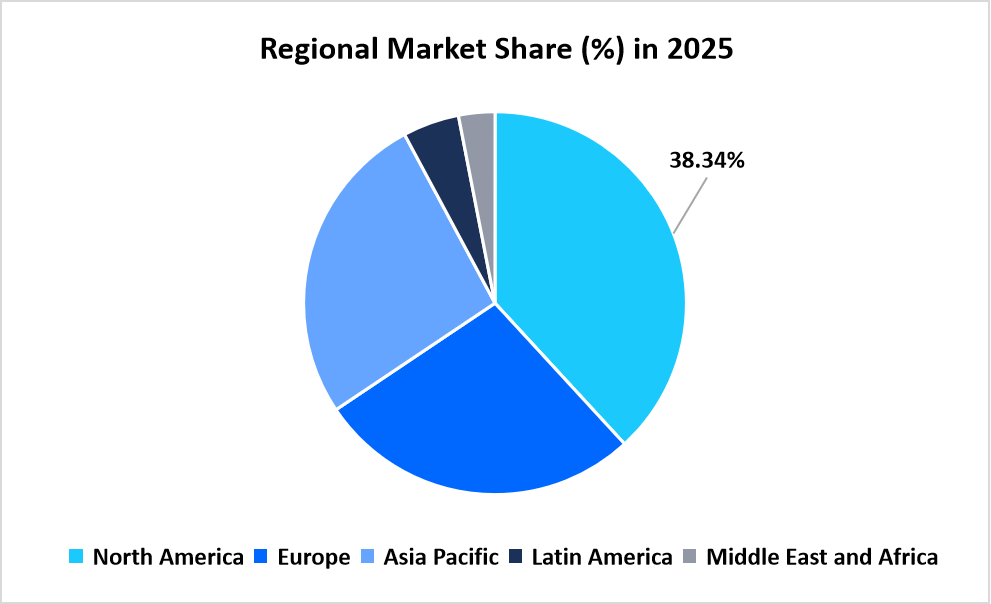
- North America held a dominant share of the global market, accounting for 38.34% share in 2025.
- The Asia Pacific region is forecasted to grow at the fastest pace, with a CAGR of 13.06%during the forecast timeframe.
- Based on Product Type, leukapheresis disposables segment is anticipated to register the fastest CAGR of 12.12% during the forecast period.
- Based on Application, therapeutics application segment is anticipated to register the fastest CAGR of 12.45% during the forecast period.
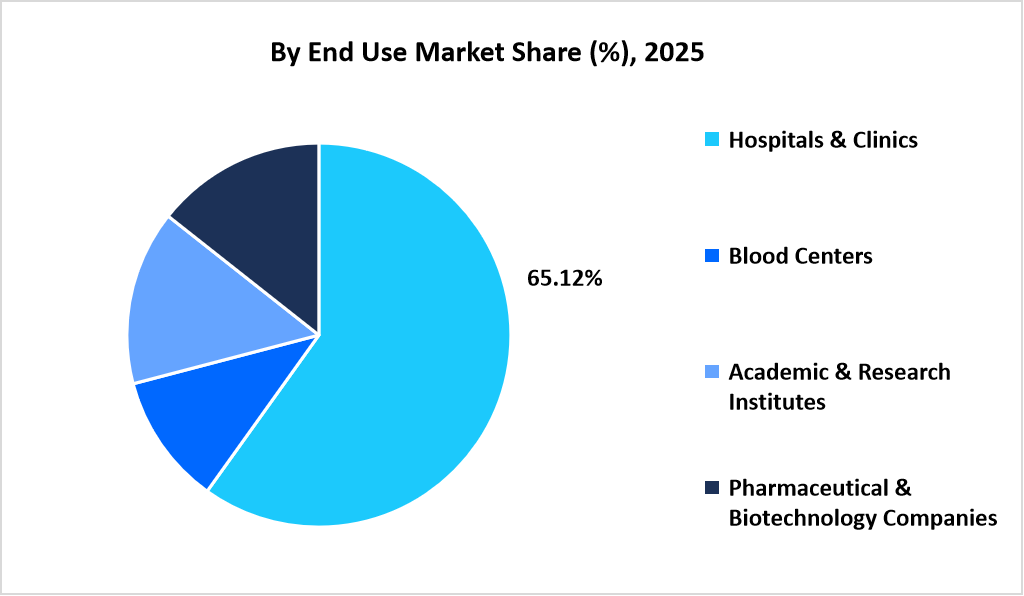
- Based on End Use, hospitals & clinics segment dominated the market with a revenue share of 65.12% in 2025.
- The U.S. dominates the market, valued at USD 73.11 million in 2024 and reaching USD 80.89 million in 2025.
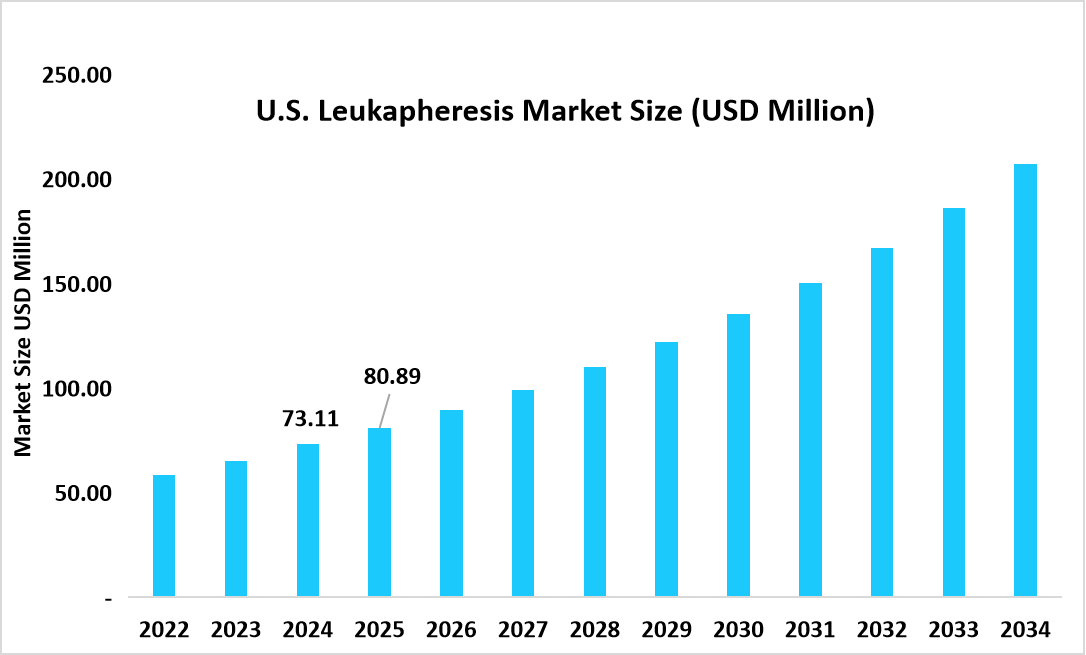
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 233.76 million
- 2034 Projected Market Size: USD 598.84 million
- CAGR (2026-2034): 11.06%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The leukapheresis market is structured across product type, application, and end use, reflecting its role in both clinical care and advanced research. By product type, the market includes leukapheresis devices, comprising centrifugal devices and membrane separators, along with leukapheresis disposables that support single-procedure use and procedural consistency. By application, leukapheresis is utilized across research and therapeutics, where research applications include cancer research, immunology research, and other investigative studies requiring high-purity leukocyte collection, while therapeutics applications focus on hematologic disorders, autoimmune diseases, and other clinical indications requiring targeted white blood cell removal or collection. By end use, the market serves blood centers, academic and research institutes, pharmaceutical and biotechnology companies, and hospitals and clinics, with hospitals and clinics acting as primary procedure sites and pharmaceutical and biotechnology companies increasingly leveraging leukapheresis as the initial step in cell and gene therapy development and manufacturing workflows.
Market Trends
Shift From Standalone Apheresis Procedures To Integrated Cell Collection Workflows
A key trend in the leukapheresis market is the transition from isolated therapeutic procedures toward integrated cell collection workflows aligned with cell and gene therapy manufacturing. Healthcare providers and therapy developers are increasingly aligning leukapheresis scheduling, sample handling, and logistics with downstream processing requirements. This coordination reduces variability in cell quality and supports smoother transfer into manufacturing pipelines, strengthening the role of leukapheresis as a foundational step in advanced therapy development.
Rising Adoption of Closed System And Single Use Leukapheresis Platforms
The notable trend is the growing preference for closed-system leukapheresis platforms supported by single-use disposables. These systems limit operator exposure and reduce contamination risk during high-volume cell collection procedures. Manufacturers are expanding disposable kit portfolios designed for specific leukocyte targets, reinforcing procedural standardization across clinical trials and commercial therapy settings.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 233.76 million |
| Estimated 2026 Value | USD 258. 77 million |
| Projected 2034 Value | USD 598.84 million |
| CAGR (2026-2034) | 11.06% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Charles River Laboratories International, Inc., Adacyte Therapeutics, AllCells, LLC, Asahi Kasei Medical, Haemonetics Corporation |
to learn more about this report Download Free Sample Report
Market Driver
Expansion of Autologous And Allogeneic Cell Therapy Pipelines
A primary driver of the leukapheresis market is the expanding pipeline of autologous and allogeneic cell therapies targeting oncology and immune-mediated disorders. Each therapy program requires consistent leukocyte collection at clinical and commercial scale, increasing procedural demand across hospitals, research centers, and manufacturing partners. This growth is reinforcing long term demand for leukapheresis devices and consumables across therapy development stages.
Market Restraint
Operational Complexity and Resource-Intensive Procedure Requirements
Leukapheresis procedures require specialized equipment, trained personnel, and controlled clinical environments. Variability in operator expertise and infrastructure availability limits procedural scalability across smaller healthcare facilities. Scheduling constraints and patient-specific factors further complicate workflow execution, creating barriers to broader procedural adoption outside specialized centers.
Market Opportunity
Integration of Leukapheresis Services Within Decentralized Therapy Manufacturing Models
A major opportunity lies in integrating leukapheresis services within decentralized and regionalized cell therapy manufacturing networks. As therapy developers shift toward localized production models, demand is rising for leukapheresis capabilities embedded within regional hospitals and partner centers. This expansion supports improved patient access, shorter vein-to-vein timelines, and sustained demand for adaptable leukapheresis platforms across emerging therapy hubs.
Regional Analysis
North America dominated the leukapheresis market in 2025 with a share of 38.34% due to the early commercialization of CAR T cell therapies and the presence of large-scale cell therapy manufacturing infrastructure. High procedural volumes across academic medical centers and oncology hospitals supported steady demand for leukapheresis systems and consumables. Reimbursement coverage for cellular therapies and the expansion of decentralized apheresis centers strengthened procedural adoption across the region.
The U.S. market growth is driven by the concentration of FDA-approved CAR T therapies and the presence of contract development and manufacturing organizations specializing in autologous cell therapies. Increasing clinical trial activity for next-generation gene-modified cell therapies has expanded leukapheresis utilization across research hospitals and commercial treatment centers.
Asia Pacific Leukapheresis Market Insights
Asia Pacific is projected to register the fastest growth of 13.06% during the forecast period, supported by rapid expansion of oncology treatment capacity and rising investments in cell and gene therapy infrastructure. Governments across the region are funding translational research programs focused on immuno-oncology, accelerating the adoption of leukapheresis procedures within hospital-based cancer centers.
The China leukapheresis industry is expanding due to the growth of domestic CAR T developers and increasing regulatory approvals for locally manufactured cell therapies. Hospital networks are investing in apheresis equipment to support both clinical trials and commercial-scale cell collection activities.
Source: Straits Research
Europe Market Insights
Europe demonstrates steady market growth driven by cross-border collaboration in cellular therapy research and harmonized regulatory pathways for advanced therapy medicinal products. Public health systems are integrating leukapheresis services within specialized hematology and transplant centers, supporting consistent procedural demand.
The Germany market benefits from a strong stem cell transplantation ecosystem and government-backed funding for immunotherapy research. The presence of specialized apheresis centers supporting both therapeutic leukapheresis and cell collection for manufacturing activities contributes to market expansion.
Latin America Market Insights
Latin America is witnessing gradual growth as tertiary care hospitals expand hematology oncology services and adopt advanced blood separation technologies. Regional demand is supported by increasing awareness of therapeutic leukapheresis for hematologic disorders and early-stage adoption of cellular therapies.
The Brazil leukapheresis market is driven by investments in public cancer institutes and the development of domestic cell therapy research programs. Collaboration between academic institutions and government research bodies is increasing access to leukapheresis procedures.
Middle East and Africa Market Insights
The Middle East and Africa market is expanding at a measured pace due to the gradual modernization of oncology treatment infrastructure and the selective adoption of advanced apheresis technologies. Demand is concentrated in specialized hospitals offering hematology and transplant services.
The Saudi Arabia market growth is supported by national healthcare transformation initiatives focused on expanding advanced cancer treatment services. Investment in specialty hospitals and medical cities is increasing access to leukapheresis procedures for complex hematologic indications.
Product Type Insights
The leukapheresis devices segment dominated the market in 2025 due to widespread deployment across hospitals and specialized apheresis centers. High utilization of centrifugal devices within therapeutic and cell collection procedures supported sustained demand, as these systems are routinely integrated into oncology and hematology treatment workflows.
The leukapheresis disposables segment is projected to grow at the fastest pace, recording a CAGR of 12.12% during the forecast period. This growth is driven by rising procedure volumes associated with CAR T cell therapy manufacturing and stem cell collection, which require single-use tubing sets, filters, and kits for each procedure, driving recurring consumption.
Application Insights
The research application segment dominated the leukapheresis market in 2025, supported by extensive use in cancer research and immunology research programs. Academic laboratories and translational research centers increasingly utilize leukapheresis to obtain high-purity leukocyte populations for cell characterization, biomarker discovery, and early-phase therapy development.
The therapeutics application segment is anticipated to grow at the fastest rate, registering a CAGR of 12.45%. Expansion of approved cellular immunotherapies for hematologic disorders and autoimmune diseases has increased the number of leukapheresis procedures performed as a prerequisite for autologous and allogeneic cell therapy production.
End Use Insights
Hospitals and clinics dominated the market in 2025, accounting for 65.12% of total revenue. This dominance is supported by centralized leukapheresis services within tertiary care hospitals that manage high patient volumes across oncology, hematology, and transplant departments.
The pharmaceutical and biotechnology companies segment is projected to grow at the fastest rate, with a CAGR of 12.67%. Growth is driven by the scale-up of clinical trials and commercial manufacturing of cell and gene therapies, where leukapheresis serves as the initial step for patient-specific cell collection used in downstream processing.
Source: Straits Research
Competitive Landscape:
The leukapheresis market is moderately competitive, with a mix of multinational medical technology companies and specialized apheresis solution providers holding notable market share.
-
Miltenyi Biotec: An emerging market player
Miltenyi Biotec is gaining attention as an emerging player in the market due to its expanding focus on cell collection and processing solutions aligned with advanced cell and gene therapy workflows. The company emphasizes integrated systems that combine leukapheresis-compatible cell separation, enrichment technologies, and closed system processing to support autologous and allogeneic therapies. Its growing collaboration with academic research centers, biotechnology firms, and clinical manufacturing facilities strengthens its positioning alongside established leukapheresis providers. By concentrating on scalable platforms, standardized consumables, and regulatory-compliant solutions, Miltenyi Biotec is steadily expanding its footprint in clinical and commercial cell therapy-driven leukapheresis applications.
List of Key and Emerging Players in Leukapheresis Market
- Charles River Laboratories International, Inc.
- Adacyte Therapeutics
- AllCells, LLC
- Asahi Kasei Medical
- Haemonetics Corporation
- Macopharma
- Cerus Corporation
- SB-Kawasumi Laboratories, Inc.
- StemExpress, LLC.
- Haemonetics Corporation
- Caltag Medsystems Limited
- Lonza Group AG
- ZenBio
- Terumo BCT, Inc.
- Macopharma SA
- Miltenyi Biotec
- Fresenius SE & Co. KGaA
- Guangzhou Daji Medical Science
- MEDICA S.p.A
- PuriBlood Medical Co., Ltd.
- Beijing ZKSK Technology Co., Ltd.
- Others
Recent Development
- March 2024: The FDA granted approval to Breyanzi, a novel CAR T-cell therapy developed by Bristol Myers Squibb, for the treatment of adults with specific forms of leukemia and lymphoma. The therapy process begins with leukapheresis, during which a patient’s white blood cells are collected and subsequently used to manufacture the personalized treatment.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 233.76 million |
| Market Size in 2026 | USD 258. 77 million |
| Market Size in 2034 | USD 598.84 million |
| CAGR | 11.06% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product type, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Leukapheresis Market Segments
By Product type
- Leukapheresis Devices
- Centrifugal Devices
- Membrane Separators
- Leukapheresis Disposables
By Application
-
Research Application
- Cancer Research
- Immunology Research
- Others
-
Therapeutics Application
- Hematologic Disorders
- Autoimmune Diseases
- Others
By End Use
- BoldoCenters
- Academic & Research Institutes
- Pharmaceutical & Biotechnology Companies
- Hospitals & Clinics
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































