Cell Therapy Market Size, Share & Trends Analysis Report By Therapy Type (Allogenic Therapies, Autologous Therapies, Non-Stem Cell Therapies), By Therapeutic Area (Oncology, Cardiovascular Disease, Musculoskeletal Disorders, Dermatology, Others), By Manufacturers (Biopharmaceutical and biotechnology companies, Pharmaceutical companies, CDMOs/CMOs, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Cell Therapy Market Overview
The global cell therapy market size was estimated at USD 6.88 billion in 2025 and is anticipated to grow from USD 8.29 billion in 2026 to USD 38.24 billion in 2034, growing at a CAGR of 21.05% from 2026-2034. The global market is experiencing a well-determined growth due to various factors, such as rapid advancements in regenerative medicine, increasing prevalence of chronic and rare diseases, and rising adoption of advanced therapeutic solutions.
Key Market Trends & Insights
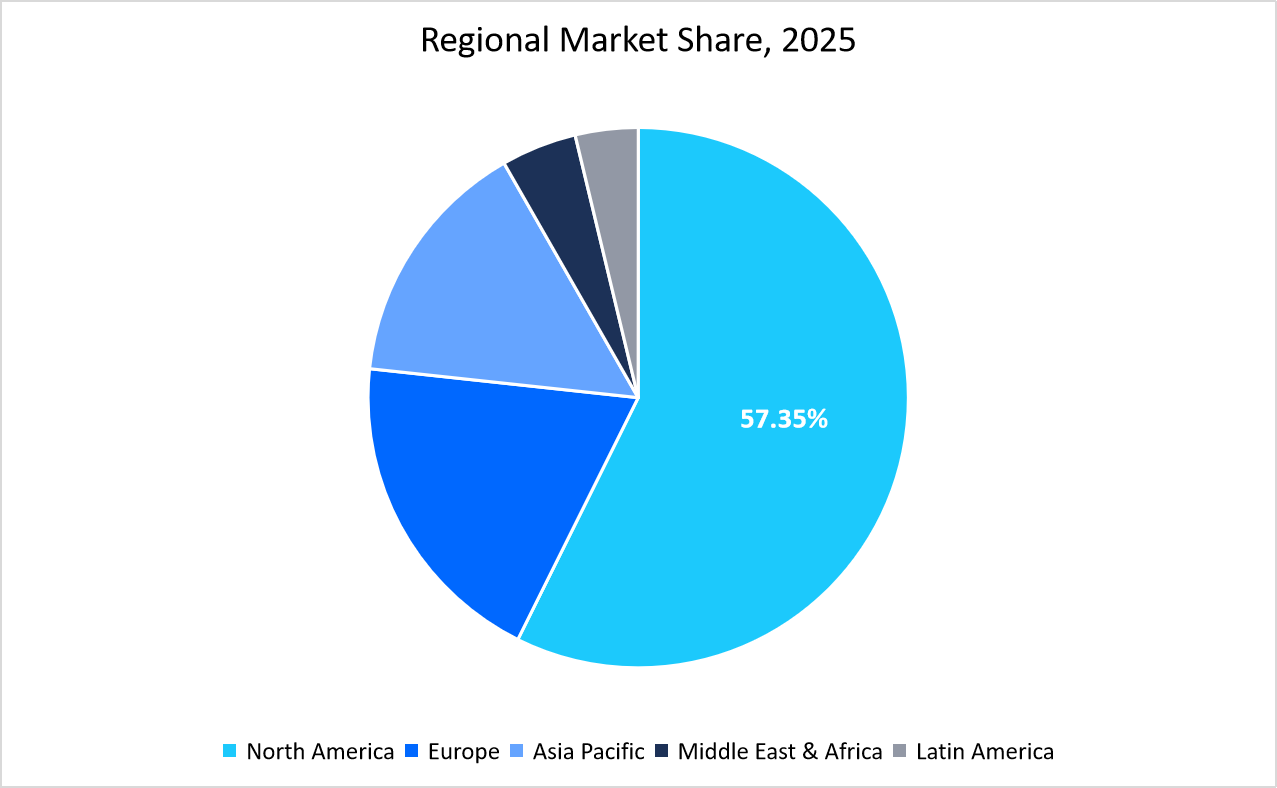
- North America held a dominant share of the global market, accounting for 57.35% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 23.76%.
- Based on the Therapy Type, the allogeneic cell therapy is expected to register the fastest CAGR of 22.65%.
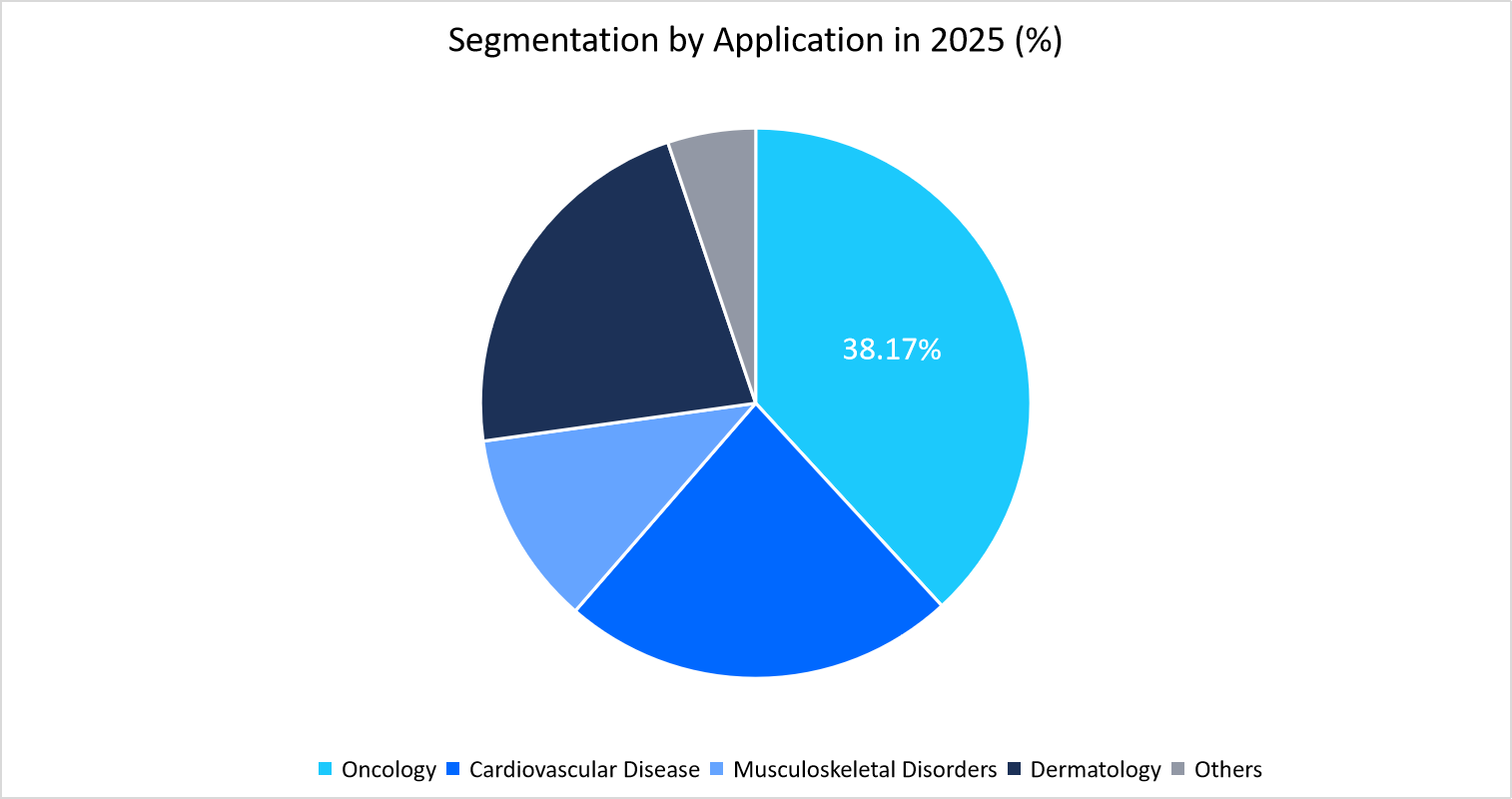
- Based on the Application, the oncology segment held the highest market share of 38.17% in 2025.
- Based on Manufacturers, the biopharmaceutical and biotechnology companies segment dominated the market in 2025.
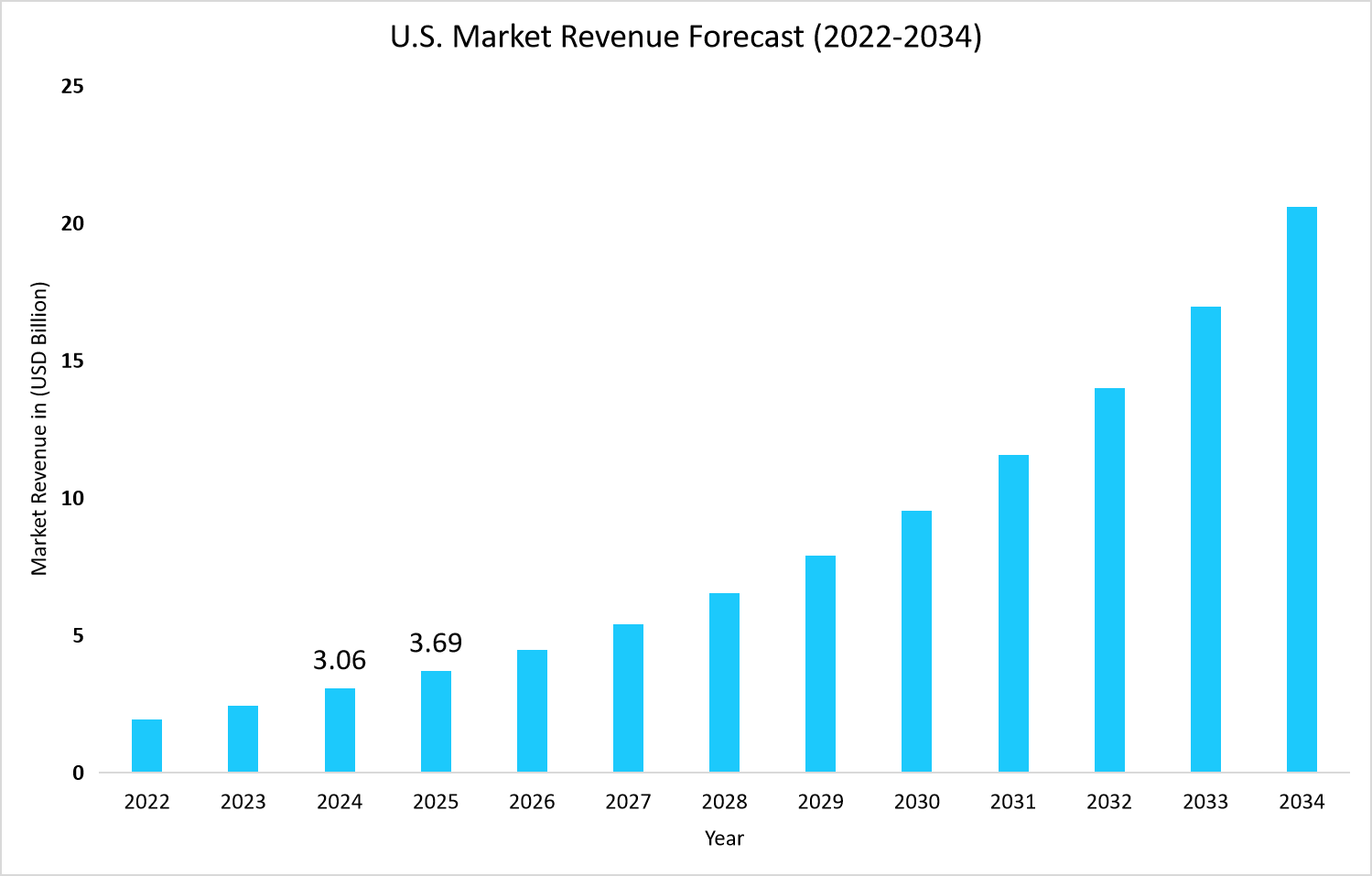
- The U.S. dominates the global cell therapy market, valued at USD 3.06 billion in 2024 and reaching USD 3.69 billion in 2025.
Market Size and Forecast
- 2025 Market Size: USD 6.88 billion
- 2034 Projected Market Size: USD 38.24 billion
- CAGR (2025 to 2034): 21.05%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
Source: Straits Research Analysis
The increasing focus on allogeneic and autologous therapies, technological innovations in cell manufacturing and delivery, supportive regulatory frameworks, and strategic collaborations among biotechnology, pharmaceutical companies, and contract development and manufacturing organizations (CDMOs), further drive the cell therapy industry growth.
Moreover, the expansion of cell therapy applications across oncology, cardiovascular, musculoskeletal, and other therapeutic areas is accelerating market adoption worldwide. Furthermore, initiatives by government agencies and non-profit organizations to improve access to innovative therapies, combined with the integration of advanced manufacturing technologies such as automation and 3D bioprinting, are further driving market growth. The growing investment in R&D, partnerships, and clinical trials for next-generation cell therapies is also strengthening the market’s long-term potential, positioning it as a transformative segment within the global healthcare industry.
Market Trends
Shift from Traditional Cell Therapy to 3D Bio-Printed, Patient-Specific Treatments
Cell therapy is moving from conventional methods, which rely on standard cell culture and transplantation, to advanced 3D bioprinting approaches that enable the fabrication of complex, patient-specific tissue structures.
- For example, in March 2024, as per the article published in Elsevier B.V., researchers at the University of California, San Diego, developed a 3D-printed scaffold seeded with stem cells to repair heart tissue damaged by myocardial infarction, demonstrating improved tissue regeneration and integration with native heart muscle.
This shift highlights a move toward more personalized and effective cell-based therapies, which further enhances the market growth.
Adoption of Advanced Stem Cell Therapies in Asia
The cell therapy landscape in Asia is evolving with the growing development and adoption of mesenchymal stem cell (MSC) therapies.
- For example, in 2025, as per the American Society of Gene + Cell Therapy, China’s NMPA approved the country’s first mesenchymal stem cell therapy, Platinum Life’s Ruibosheng, indicated for steroid-refractory acute graft-versus-host disease.
Such a factor reflects a broader trend of integrating advanced stem cell therapies into clinical practice across the region, expanding treatment options for patients with previously limited alternatives.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 6.88 billion |
| Estimated 2026 Value | USD 8.29 billion |
| Projected 2034 Value | USD 38.24 billion |
| CAGR (2026-2034) | 21.05% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Novartis AG, Amgen Inc., Vericel Corporation, Gilead Sciences, Inc., Bluebird Bio, Inc. |

to learn more about this report Download Free Sample Report
Market Drivers
Expansion of Manufacturing Capacity for Autologous Therapies
The growth of autologous cell therapies is supported by the expansion of manufacturing capabilities, which presents as a significant driver for market growth.
- For example, in February 2024, AstraZeneca announced a $300 million project to establish a new cell-therapy facility in Rockville, Maryland, focused on producing T-cell therapies for clinical and commercial supply.
Such facility enhances production capacity, improves supply reliability, and facilitates wider access to autologous therapies.
Outcomes-Based Access for Cell Therapies
Cell therapies are highly expensive, often costing hundreds of thousands of dollars per patient, which creates major reimbursement and access challenges. To address this, outcomes-based payment models are being adopted, where reimbursement is directly tied to the therapy’s effectiveness in improving patient health.
- For example, in March 2024, the Centers for Medicare & Medicaid Services (CMS) launched the Cell and Gene Therapy (CGT) Access Model, enabling value-based agreements to expand access while controlling costs.
This approach encourages the broader adoption of innovative cell therapies, which further enhances the market growth.
Market Restraint
High Cost and Manufacturing Complexity of Advanced Therapies
High cost and manufacturing complexity of advanced therapies, hinders the market growth as it limits the patient access.
- For example, in April 2025, the UK’s National Institute for Health and Care Excellence (NICE) initially declined to recommend BMS’s Abecma (idecabtagene vicleucel) for multiple myeloma due to concerns over its high price relative to clinical benefit.
Such factor highlights the ongoing affordability and reimbursement challenges, which further restrains the market growth.
Market Opportunities
Expanding the Scope of Cell Therapy Via the Expansion of Allogeneic Car-T Platforms
Expansion of allogeneic CAR-T platforms, presents as a significant market opportunity as it offers scalable, off-the-shelf treatments.
- For example, in January 2025, Allogene Therapeutics announced that the U.S. FDA cleared its Investigational New Drug (IND) application for ALLO-329, an allogeneic CAR-T candidate targeting autoimmune diseases.
Such factor acts as a significant step in expanding the scope of cell therapy applications beyond oncology into immune-mediated disorders.
Regional Insights
The North America region dominated the market with a revenue share of 57.35 % in 2025. The growth is attributed to factors such as strong presence of leading biotech and pharmaceutical companies, rapid adoption of CAR-T and stem cell therapies, supportive regulatory pathways from the U.S. FDA, significant R&D investments, and extensive clinical trial activity supported by advanced healthcare infrastructure and academic-industry collaborations.
Asia Pacific Market Trends
The Asia Pacific region is the fastest-growing region with a CAGR of 23.76 % during the forecast timeframe. The growth is attributed to factors such as a larger patient population, increasing government initiatives to strengthen biotechnology infrastructure, rising investment in advanced therapies by regional manufacturers, and rapid expansion of GMP-certified cell facilities.
Source: Straits Research Analysis
Country Analysis
U.S. Market Trends
The cell therapy industry in U.S. is widely driven by improved patient access, supported by regulatory as well as payer progress. For example, in July 2025, as per the U.S. Centers for Medicare & Medicaid Services, 84% of Medicaid beneficiaries with sickle cell disease are in states participating in CMS’s Cell therapy (CGT) Access Model.
UK Market Growth Factors
The market in the UK is propelling due to the rapid expansion of GMP manufacturing capacity, NHS adoption of CAR-T therapies and new regulations to enhance the manufacturing of cell therapies. For example, in July 2025, as per the Association for the Advancement of Blood and Biotherapies, the U.K.’s Medicines and Healthcare products Regulatory Agency (MHRA) introduced “The Human Medicines (Amendment) (Modular Manufacture and Point of Care) Regulations 2025” for the on-site manufacture of advanced therapies, including cell therapies.
Germany Market Trends
In Germany, the market is driven by the strong industry and government initiatives. For example, in June 2024, as per DZIF, the government launched the National Strategy for Gene and Cell-Based Therapies to provide regulatory guidance and improve patient access nationwide.
China Market Growth Factors
The cell therapy market in China is fuelled by the strong manufacturing investments as well as regulatory reforms and focus of companies towards the development of advanced therapies. For example, in June 2025, CARsgen Therapeutics Holdings Limited announces that the National Medical Products Administration (NMPA) of China has accepted the New Drug Application (NDA) for satricabtagene autoleucel, an autologous CAR-T cell product for the treatment of Claudin18.2 in patients who have failed at least two prior lines of therapy.
India Market Trends
India continues to position itself in the cell therapy market, due to the rise of affordable home-grown CAR-T treatments. For example, in April 2024, as per the PIB, India launched its first homegrown genetic therapy, NexCAR19, developed via the collaborations between IIT Bombay, Tata Memorial Centre, and ImmunoACT.
Therapy Type Insights
The allogeneic cell therapy segment is anticipated to grow at a CAGR of 22.65 % from 2026 TO 2034. The growth is attributed to its scalability, off-the-shelf availability, and the ability to treat multiple patients from a single donor batch, which reduces production time and cost.
Application Insights
The oncology segment dominates the market with a revenue share of 38.17% in 2025. This growth is attributed to the strong pipelines as well as extensive clinical trials in oncology, which continue to attract the highest investments and collaborations globally.
Source: Straits Research Analysis
Manufacture Insights
The Biopharmaceutical and biotechnology companies segment dominated the market in 2025. This growth is attributed to their active collaborations with academic institutions and healthcare providers, the presence of supportive regulatory frameworks that enable faster product approvals, and the rising demand for personalized therapies.
Competitive Landscape
The global cell therapy market is highly fragmented in nature due to presence of numerous biotechnology startups, academic spin-offs, and mid-sized companies alongside a few major pharmaceutical players such as Novartis AG, Gilead Sciences, Inc and Bristol-Myers Squibb Company, Bluebird Bio, Inc., and Others.
The industry participants are inclined towards adopting key business strategies, such as strategic collaborations, product approvals, acquisitions, and product launches, to gain a strong foothold in the cell therapy market.
Phoenix Senolytix.: An Emerging Market Player
Phoenix SENOLYTIX is a company that uses advanced technologies to develop novel gene therapies.
- In September 2025, Phoenix SENOLYTIX and the University of Texas MD Anderson Cancer Center in the U.S. collaborated to advance the progress of inducible switch technologies for cell therapy applications.
List of Key and Emerging Players in Cell Therapy Market
- Novartis AG
- Amgen Inc.
- Vericel Corporation
- Gilead Sciences, Inc.
- Bluebird Bio, Inc.
- Bristol-Myers Squibb Company
- Johnson & Johnson and its affilates
- Celgene Corporation
- ImmunoACT
- Cellectis
- Astellas Pharma Inc.
- Atara Biotherapeutics, Inc.
- Sana Biotechnology
- Bayer AG
- F. Hoffmann-La Roche Ltd
- ALLOGENE THERAPEUTICS
- Intas Pharmaceuticals Ltd.
- EUREKA THERAPEUTICS
- Novo Nordisk A/S
- Kyverna Therapeutics, Inc.
to learn more about this report Download Market Share
Recent Developments
- May 2025: Astraveus SAS entered into a strategic partnership with the Netherlands Center for the Clinical Advancement of Stem Cell and Gene Therapies (NecstGen) to evaluate the Lakhesys Benchtop Cell Factory for the manufacturing of CAR-T therapies.
- April 2025: XellSmart Biopharmaceutical Co., Ltd. announced that the U.S. FDA cleared its universal allogeneic off-the-shelf iPS-derived dopaminergic neural progenitor cell therapy for the treatment of Parkinson's disease, which is considered the second most common neurodegenerative disease worldwide.
Analyst Opinion
The global cell therapy market is witnessing robust momentum, driven by increasing clinical successes in CAR-T therapies, expanding applications of stem cell treatments, and supportive regulatory pathways. Emerging biotech’s are pushing innovation with iPSC-derived and allogeneic platforms that promise scalability and cost-efficiency, while established players accelerate commercialization. Furthermore, investment inflows, and expanding trial pipelines position the cell therapy sector as a transformative frontier in precision medicine and long-term healthcare innovation.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 6.88 billion |
| Market Size in 2026 | USD 8.29 billion |
| Market Size in 2034 | USD 38.24 billion |
| CAGR | 21.05% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Therapy Type, By Therapeutic Area, By Manufacturers |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Singapore, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Cell Therapy Market Segments
By Therapy Type
-
Allogenic Therapies
- Stem Cell Therapies
- Hematopoietic Stem Cell Therapies
- Mesenchymal Stem Cell Therapies
- Non-Stem Cell Therapies
- Keratinocytes & Fibroblast based therapies
- Others
-
Autologous Therapies
- Stem Cell Therapies
- BM, Blood, & Umbilical Cord-derived Stem Cells
- Adipose Derived Cells
- Others
-
Non-Stem Cell Therapies
- T Cell Therapies
- CAR T Cell Therapy
- T cell Receptor based
- Others
By Therapeutic Area
- Oncology
- Cardiovascular Disease
- Musculoskeletal Disorders
- Dermatology
- Others
By Manufacturers
- Biopharmaceutical and biotechnology companies
- Pharmaceutical companies
- CDMOs/CMOs
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Dhanashri Bhapakar
Senior Research Associate
Dhanashri Bhapakar is a Senior Research Associate with 3+ years of experience in the Biotechnology sector. She focuses on tracking innovation trends, R&D breakthroughs, and market opportunities within biopharmaceuticals and life sciences. Dhanashri’s deep industry knowledge enables her to provide precise, data-backed insights that help companies innovate and compete effectively in global biotech markets.










































