Liver Disease Therapeutics Market Size, Share & Trends Analysis Report By Product (Vaccines, Antiviral Drugs, Chemotherapy, Targeted Therapy, Immunosuppressants, Corticosteroids, Others), By Disease (Hepatitis, Non-alcoholic Fatty Liver Disease (NAFLD), Hepatic Cancer, Genetic Disorders, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Liver Disease Therapeutics Market Overview
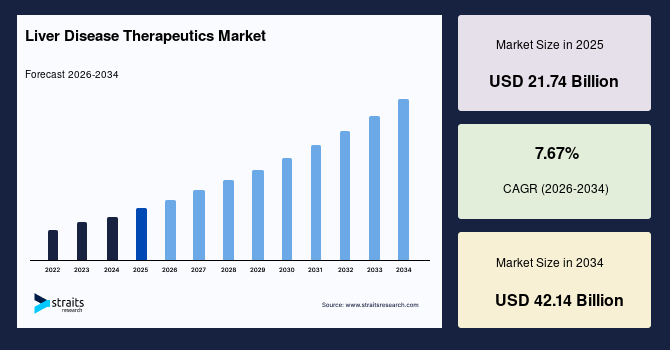
The global liver disease therapeutics market size is valued at USD 21.74 billion in 2025 and is estimated to reach USD 42.14 billion by 2034, growing at a CAGR of 7.67% during the forecast period. The consistent growth of the market is supported by the rapid advancement of RNA interference and gene-silencing therapies specifically targeting hepatic pathways.
Key Market Trends & Insights
- North America dominated the global market, accounting for a 38.92% share in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 9.83% during the forecast period.
- Based on product, the antiviral drugs segment dominated the market in 2025, with a revenue share of 36.74% in 2025.
- Based on disease, the hepatitis segment dominated the market with a revenue share of 41.03% in 2025.
- S. dominates the liver disease therapeutics market, valued at USD 7.11 billion in 2024 and reaching USD 7.62 billion in 2025.
Table: U.S. Liver Disease Therapeutics Market Size (USD Million)
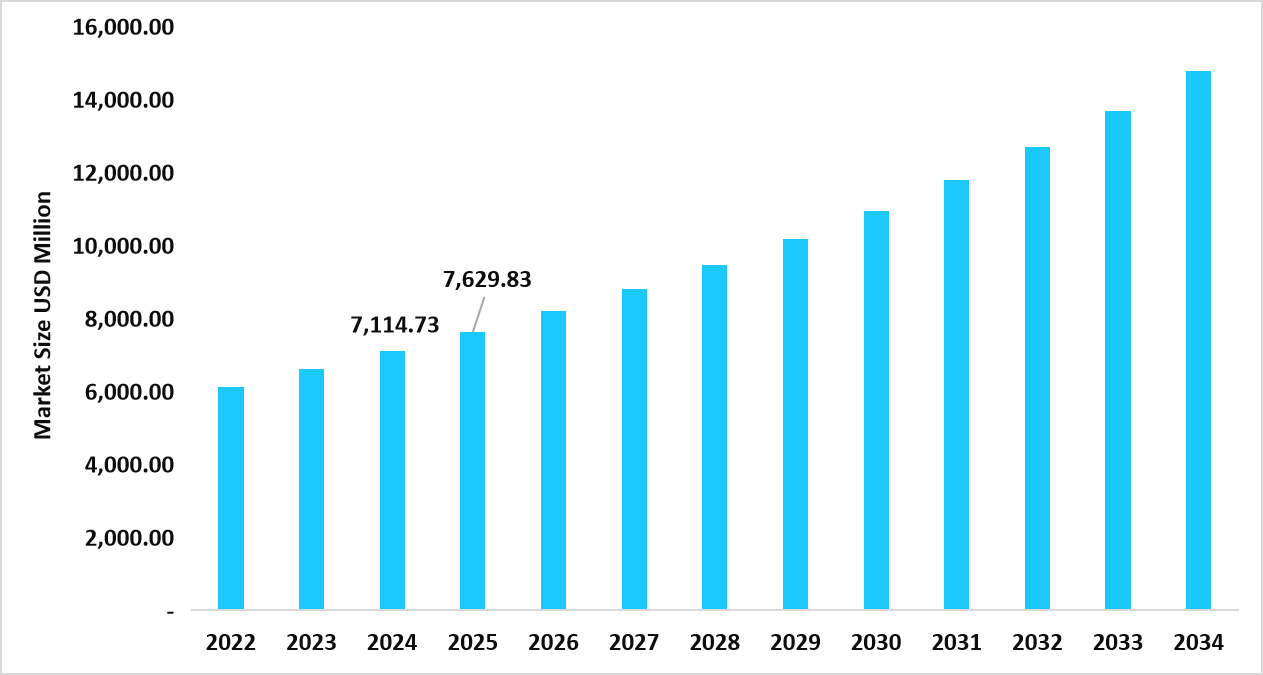
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 21.74 billion
- 2034 Projected Market Size: USD 42.14 billion
- CAGR (2026-2034): 7.67%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The global liver disease therapeutics market encompasses a diverse range of treatment modalities, including vaccines, antiviral drugs, chemotherapy, targeted therapies, immunosuppressants, corticosteroids, and other supportive treatments. These therapeutics are utilized across multiple liver disease indications such as hepatitis, non-alcoholic fatty liver disease, hepatic cancer, genetic liver disorders, and related conditions, addressing both disease management and progression prevention across varied clinical settings.
Latest Market Trends
Shift Toward Disease-modifying and RNA-based Therapies
A major trend in the liver disease therapeutics market is the transition from symptomatic management to disease-modifying treatments, particularly for NASH/MASH and chronic hepatitis. The approval and late-stage development of thyroid hormone receptor-β agonists, RNA interference therapies, and antisense oligonucleotides reflect this shift.
These advanced modalities target underlying hepatic pathways, improving fibrosis and metabolic outcomes, and are reshaping clinical practice and investment priorities.
Expansion of Specialized Hepatology Centers and Outpatient Liver Clinics
The expansion of specialized hepatology centers and outpatient liver clinics is a notable trend influencing the liver disease therapeutics market. In the U.S. and Europe, healthcare systems are increasingly establishing dedicated liver care units to manage chronic conditions such as NASH, hepatitis, and cirrhosis. Notably, following the 2024 approval of the first NASH therapy, several academic hospitals expanded outpatient hepatology programs, improving early diagnosis, treatment initiation, and patient adherence, thereby accelerating therapeutic adoption.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 21.74 Billion |
| Estimated 2026 Value | USD 23.33 Billion |
| Projected 2034 Value | USD 42.14 Billion |
| CAGR (2026-2034) | 7.67% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | AbbVie Inc., Alnylam Pharmaceuticals, Inc., Arrowhead Pharmaceuticals, Inc., Astellas Pharma Inc., Bayer AG |
to learn more about this report Download Free Sample Report
Market Drivers
Rising Prevalence of Metabolic and Viral Liver Disorders
The growing incidence of metabolic and viral liver disorders is a major driver of the liver disease therapeutics market. Non-alcoholic fatty liver disease now affects approximately one quarter of the global adult population, while chronic hepatitis B and C remain highly prevalent across Asia Pacific and Africa. In 2024, various countries expanded national hepatitis screening and treatment programs, notably increasing patient diagnosis rates and therapeutic uptake, thereby accelerating market growth.
Market Restraint
High Cost of Advanced Liver Disease Therapies
The high cost of advanced liver disease therapeutics remains a major restraint on market growth. Innovative treatments such as direct acting antivirals, biologics, and emerging RNA-based therapies carry annual treatment costs exceeding USD 20,000 to 80,000 per patient, depending on disease severity and therapy duration. Limited reimbursement coverage, especially in low and middle-income countries, further restricts patient access.
These cost barriers impact treatment affordability, slowing widespread adoption despite strong clinical efficacy.
Market Opportunity
Strong Late-stage Pipeline for Nash and Fibrosis Therapies
The robust late-stage clinical pipeline targeting NASH/MASH and liver fibrosis represents a major market opportunity. As of 2025, over 30 drug candidates are in Phase II and Phase III development globally, focusing on mechanisms such as THR-β agonists, FXR agonists, and anti-fibrotic pathways. The absence of multiple approved therapies and the large untreated patient base create substantial commercial potential, encouraging sustained investment and long-term market expansion.
Regional Analysis
North America dominated the liver disease therapeutics market in 2025, accounting for 38.92% market share. This growth is augmented by its established public health programs for hepatitis screening and treatment in the U.S., which facilitate early diagnosis and timely therapy initiation. This proactive approach increases patient access to antivirals and advanced liver therapies, driving sustained regional market expansion.
Canada market growth is driven by the wide public and private reimbursement of GIVLAARI (givosiran), an RNA interference therapy for acute hepatic porphyria, ensuring funded access nationwide. This broad coverage for an ultra-rare liver condition enhances uptake of advanced liver therapeutics and signals expanding payer support for innovative treatment modalities in the Canadian market.
Asia Pacific Market Insights
Asia Pacific is emerging as the fastest-growing region with a CAGR of 9.83% from 2026 to 2034, owing to the expansion of government‑backed hepatitis treatment financing and universal access programs, where multiple countries now include HBV and HCV therapies under national insurance or public funding schemes. This reduces out‑of‑pocket costs, increases treatment uptake, and strengthens therapeutic demand across large patient populations.
Australia's liver disease therapeutics market growth is supported by the inclusion of new chronic liver medicines like Iqirvo (elafibranor) on the Pharmaceutical Benefits Scheme (PBS), markedly reducing patient costs to under AUD 40 per prescription. This government subsidy dramatically improves therapy affordability and accessibility, driving higher treatment uptake across chronic liver disease populations nationwide.
Regional Market share (%) in 2025
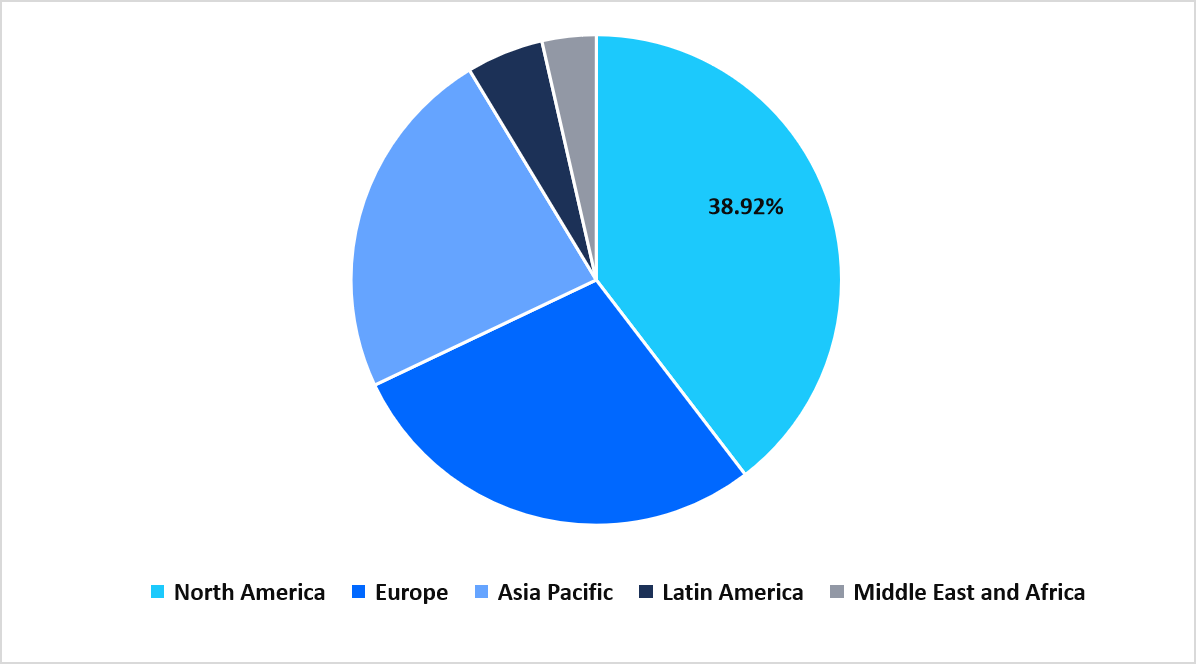
Source: Straits Research
Europe Market Insights
Europe's liver disease therapeutics market is boosted by the region’s conditional authorisation mechanism to expedite access to innovative liver therapies, such as the conditional approval recommendation for Rezdiffra for NASH/MASH. This regulatory flexibility allows earlier market availability of breakthrough treatments, encouraging clinician adoption and investment in advanced liver therapeutics across the region.
In Germany, the market is experiencing notable growth, driven by a high concentration of advanced clinical trial activity and hepatology expertise within leading university hospitals, especially in Berlin, Munich, and Heidelberg. This robust research ecosystem accelerates early adoption of innovative therapies and supports evidence-based treatment protocols across complex liver conditions.
Latin America Market Insights
Latin America’s liver disease therapeutics market is being driven by the wider availability of generic direct-acting antiviral (DAA) formulations through regional procurement agreements and government programs, which have reduced treatment costs and expanded access across Brazil, Mexico, and Argentina, facilitating broader therapy adoption in underserved populations.
The growth of Argentina's market is driven by the government’s long-standing policy of incorporating direct‑acting antiviral (DAA) treatments for hepatitis B and C into the public health system free of charge, expanding access regardless of fibrosis stage, and improving treatment penetration nationwide.
Middle East and Africa Market Insights
The Middle East and Africa liver disease therapeutics market growth is stimulated by the region’s exceptionally high prevalence of non‑alcoholic fatty liver disease, particularly in Gulf states, driven by rapid lifestyle changes and metabolic risk factors. This elevated disease burden increases clinical demand for advanced liver therapies and accelerates market uptake across the region.
South Africa market growth is supported by the accelerating adoption of non-invasive screening for NAFLD and NASH, integrated into routine diabetes and obesity care pathways. This increased early detection has expanded identification of at‑risk patients eligible for therapeutic intervention, boosting demand for pharmacological treatments in clinical practice.
Product Insights
The antiviral drugs segment dominated the market in 2025, accounting for 36.74% revenue share. This growth is driven by the increasing clinical preference for genotypic antiviral regimens that eliminate the necessity for genotype testing, notably reducing diagnostic complexity, treatment initiation time, and healthcare system burden, especially in high-volume public treatment programs.
The targeted therapy segment is estimated to register the fastest CAGR of 8.71% during the forecast period, owing to the rising development of liver-selective molecular therapies engineered to minimize systemic exposure, enabling higher dosing precision and improved safety profiles, which is driving faster clinical adoption across chronic and progressive liver disease indications.
By Product Market Share (%), 2025
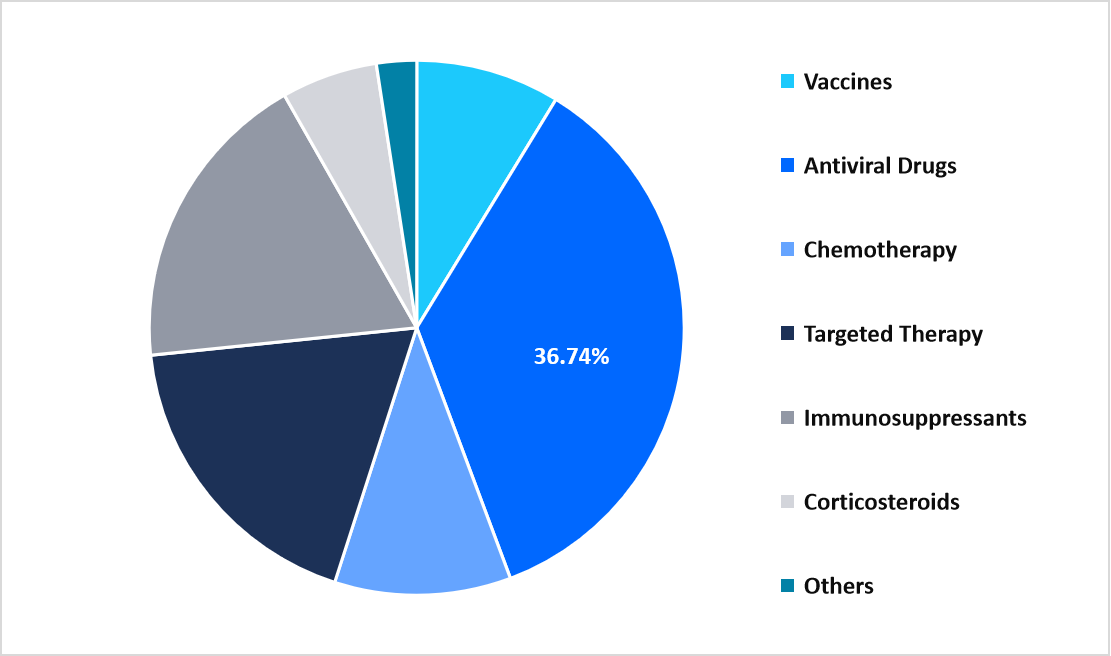
Source: Straits Research
Disease Insights
The hepatitis segment dominated the market with the highest revenue share of 41.03% in 2025. This growth is attributed to the high recurrence of treatment-experienced hepatitis patients requiring retreatment with antivirals, creating sustained demand within the hepatitis disease segment beyond first-line therapy initiation.
The non-alcoholic fatty liver disease (NAFLD) segment is projected to register the fastest CAGR of 8.76% from 2026-2034, due to rising focus on therapies targeting hepatic lipid metabolism and insulin resistance, which directly address the underlying pathophysiology of NAFLD, driving increased adoption of novel pharmacological interventions in this patient population.
Competitive Landscape
The global liver disease therapeutics market is moderately consolidated, with several multinational pharmaceutical and biotech companies leading key segments. Market leaders maintain their position through continuous R&D, strategic partnerships, and regulatory approvals. Prominent players include Gilead Sciences, AbbVie, Bristol-Myers Squibb, Roche, Merck, Intercept Pharmaceuticals, Alnylam Pharmaceuticals, and others, focusing on innovative antivirals, targeted therapies, and RNA-based treatments to address chronic liver conditions worldwide.
Tune Therapeutics: An Emerging Market Player
Tune Therapeutics is an emerging biotech company developing a novel epigenetic gene tuning therapy for chronic hepatitis B, aiming to disable viral replication in liver cells with a single injectable treatment.
- In early 2025, it raised $175 million to advance clinical trials across multiple regions, highlighting its potential to transform treatment for persistent viral liver infections and address unmet therapeutic demand in the market.
List of Key and Emerging Players in Liver Disease Therapeutics Market
- AbbVie Inc.
- Alnylam Pharmaceuticals, Inc.
- Arrowhead Pharmaceuticals, Inc.
- Astellas Pharma Inc.
- Bayer AG
- Bristol‑Myers Squibb Company
- Merck & Co., Inc.
- CymaBay Therapeutics, Inc.
- Hoffmann‑La Roche Ltd.
- Gilead Sciences, Inc.
- GSK plc
- Intercept Pharmaceuticals, Inc.
- Johnson & Johnson Services, Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Ionis Pharmaceuticals, Inc.
- Conatus Pharmaceuticals, Inc.
- Others
Strategic Initiatives
- August 2025: The U.S. Food and Drug Administration (FDA) approved Wegovy injection developed by Novo Nordisk to treat metabolic-associated steatohepatitis (MASH).
- March 2025: Japan’s Ministry of Health approved Takeda’s Livmarli (maralixibat) Oral Solution for treating Alagille syndrome and PFIC, reinforcing the company’s commitment to rare pediatric liver diseases.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 21.74 Billion |
| Market Size in 2026 | USD 23.33 Billion |
| Market Size in 2034 | USD 42.14 Billion |
| CAGR | 7.67% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Disease |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Liver Disease Therapeutics Market Segments
By Product
- Vaccines
- Antiviral Drugs
- Chemotherapy
- Targeted Therapy
- Immunosuppressants
- Corticosteroids
- Others
By Disease
- Hepatitis
- Non-alcoholic Fatty Liver Disease (NAFLD)
- Hepatic Cancer
- Genetic Disorders
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































