Mitochondrial DNA (mtDNA) Market Size, Share & Trends Analysis Report By Product Type (mtDNA-Based Fertility Testing Kits, Next-Generation Sequencing (NGS)-Based Kits, PCR-Based mtDNA Testing Kits, Whole Mitochondrial Genome Panels, Bioinformatics Analysis Software), By Application (Embryo Viability and Donor Egg Screening , Fertility and Reproductive Health Diagnostics, Mitochondrial Disorder Screening, Ancestry and Genealogy Research, Cancer Biomarker Detection, Neurodegenerative and Metabolic Disease Studies), By Distribution Channel (Fertility Clinics and IVF Centers, Hospitals and Diagnostic Laboratories, Genetic Testing Companies (e.g., 23andMe, MyHeritage), Academic & Research Institutions, Direct-to-Consumer (DTC) Platforms, Government and Public Health Agencies), By End-User (Reproductive endocrinologists, Clinical Diagnostics Laboratories, Pharmaceutical & Biotech Companies, Academic & Genomic Research Centers) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Mitochondrial DNA (mtDNA) Market Overview
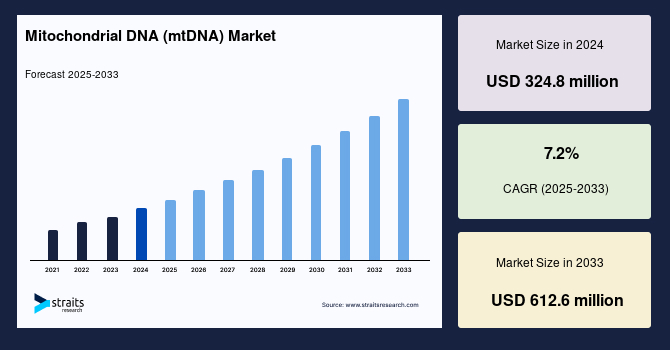
The global mitochondrial DNA (mtDNA) market size was valued at USD 324.8 million in 2024 and is projected to grow from USD 347.2 million in 2025 to USD 612.6 million by 2033, registering a CAGR of 7.2% during the forecast period (2025–2033). The growth of the market is attributed to integrated functional and genetic testing enhances mtDNA diagnostic accuracy.
Key Market Indicators
- North America dominated the mitochondrial DNA (mtDNA) industry and accounted for a 41.2% share in 2024.
- Based on product type, the mtDNA-based fertility testing kits segment held the largest share of the mitochondrial DNA (mtDNA) market in 2024, owing to increasing infertility rates and the role of mtDNA in embryo quality.
- Based on application, the embryo viability and donor egg Screening segment is witnessing steady growth, driven by the rising use of mtDNA biomarkers to assess embryo health.
- Based on distribution channel, the fertility clinics and IVF centers segment dominated the mitochondrial DNA (mtDNA) industry in 2024 due to the rising preference for assisted reproductive technologies (ART).
Market Size & Forecast
- 2024 Market Size: USD 324.8 Million
- 2033 Projected Market Size: USD 612.6 Million
- CAGR (2025-2033): 7.2%
- North America: Largest market in 2024
The global mitochondrial DNA (mtDNA) market is witnessing robust growth, propelled by the expanding applications of mtDNA analysis in medical diagnostics, ancestry tracing, forensic science, and evolutionary biology. Mitochondrial DNA (mtDNA) found in the cell’s mitochondria and inherited maternally plays a crucial role in ancestry tracing, diagnosing mitochondrial disorders, and detecting mutations linked to diseases like cancer and neurodegenerative conditions. The global mtDNA market is expanding rapidly, driven by the growing availability of high-throughput and next-generation sequencing (NGS) technologies that enhance analysis speed, accuracy, and cost-efficiency. Rising awareness of mitochondrial diseases, increased adoption of personalized medicine, and expanding genetic research are boosting demand.
Market Trend
Constraint Metrics Enhance Variant Prioritization in Mtdna Diagnostics
The mitochondrial DNA (mtDNA) market is being significantly advanced by new data-driven tools that improve the clinical interpretation of genetic variants, thereby accelerating diagnostic accuracy and therapeutic insights. One of the most impactful recent trends is the introduction of constraint metrics that pinpoint functionally critical regions of the mitochondrial genome.
- For instance, in February 2025, the Genome Aggregation Database (gnomAD) released new mitochondrial genome constraint metrics, developed using mtDNA data from over 56,000 individuals. These metrics identify regions under strong evolutionary pressure, suggesting high functional importance, and provide both gene-level and regional constraint scores.
The availability of this large-scale, curated population dataset is a major boost to diagnostic laboratories and genetic testing companies working in the mtDNA space. It supports improved interpretation pipelines, reduces false positives, and enhances personalized medicine applications by helping focus on clinically relevant mutations.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 324.8 million |
| Estimated 2025 Value | USD 347.2 million |
| Projected 2033 Value | USD 612.6 million |
| CAGR (2025-2033) | 7.2% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Illumina, Inc., Thermo Fisher Scientific Inc., F. Hoffmann-La Roche Ltd, QIAGEN N.V., BGI Genomics Co., Ltd. |
to learn more about this report Download Free Sample Report
Market Driver
Integrated Functional and Genetic Testing Enhances Mtdna Diagnostic Accuracy
A major driver accelerating the mitochondrial DNA (mtDNA) market in 2025 is the advancement of diagnostic methods that combine functional and genetic testing to improve the detection of mitochondrial disorders, especially in complex or low-heteroplasmy cases.
- For example, in March 2025, Baylor Genetics presented new clinical data at the ACMG Annual Clinical Genetics Meeting, demonstrating the effectiveness of integrating electron transport chain (ETC) functional assays with long-range PCR and next-generation sequencing (NGS). Based on a retrospective study of 1,118 muscle tissue samples, the findings revealed that low-heteroplasmy, single large-scale mtDNA deletions often undetected by sequencing alone were reliably identified through combined enzyme-based and genetic testing approaches.
By improving the sensitivity and reliability of mtDNA variant detection, especially in pediatric neuromuscular cases, this approach is shaping how hospitals, specialty labs, and research centers develop and adopt mitochondrial testing platforms.
Market Restraint
Interpretation Complexity and Low-Heteroplasmy Detection Pose Diagnostic Challenges
The ongoing difficulty in accurately interpreting variants, especially in cases involving low heteroplasmy levels or ambiguous clinical relevance, restricts the market. The lack of standardized interpretation guidelines often results in variants of uncertain significance (VUS), which limit clinical decision-making and patient confidence. Low-heteroplasmy mutations, where the mutated mtDNA represents a small proportion of total mtDNA, can be especially challenging to detect and validate.
Another limiting factor is the shortage of high-quality reference databases and constraint metrics specific to mtDNA compared to nuclear DNA, making it harder for clinicians and geneticists to classify pathogenicity confidently. Additionally, high costs and lack of reimbursement for advanced mtDNA testing in many countries reduce accessibility, especially in resource-constrained healthcare settings.
Market Opportunity
Mitochondrial Replacement Therapy (mrt) Drives Long-Term Growth in Mtdna Testing and Counseling
One of the most consequential opportunities for the mtDNA market in 2025 is the expanding role of mitochondrial replacement therapy (MRT), a reproductive technique designed to prevent the transmission of severe mitochondrial diseases by replacing faulty maternal mitochondria with healthy donor mitochondria.
- For instance, in July 2025, UK researchers at Newcastle University reported the successful birth of eight healthy babies conceived using MRT, which was legally approved in 2015. The technique allows women carrying pathogenic mtDNA mutations to have genetically related children free of mitochondrial disease symptoms. These were among the first MRT births globally, marking a pivotal milestone in clinical reproductive genetics.
As clinics globally consider adopting MRT, both patients and providers will require accurate diagnostic tools, variant interpretation platforms, and ethical-genetic advisory frameworks.
Regional Analysis
North America holds a dominant position in the mtDNA fertility diagnostics market, supported by a mature ecosystem for reproductive medicine and strong adoption of genomic technologies in fertility treatments. The region benefits from advanced IVF infrastructure, widespread availability of mitochondrial screening services, and a high level of clinician awareness. Industry players are investing in AI-powered mtDNA platforms, enabling more personalized embryo selection and improved IVF outcomes. Growing clinical interest in mitochondrial therapeutics, along with favorable policies for genetic testing and fertility care coverage, further strengthens the region’s dominance in this space. Collaborations between academic institutions and fertility tech companies are accelerating innovation, while consumer demand for proactive fertility planning drives early-stage testing and awareness campaigns.
U.s. Mitochondrial Dna Market Trends
- The U.S. is a global leader in mtDNA fertility diagnostics, propelled by an advanced IVF ecosystem and growing acceptance of mitochondrial medicine. Fertility clinics are increasingly adopting mtDNA-based embryo viability scoring, heteroplasmy analysis, and oocyte quality screening. Regulatory bodies like the FDA are actively reviewing Mitochondrial Replacement Therapy (MRT), while major research networks drive innovation in non-invasive mitochondrial assays and genomic profiling. Strong venture capital interest and biotech start-up activity further position the U.S. at the cutting edge of this domain. Recent clinical trials involving AI-integrated mtDNA scoring tools are enhancing embryo selection accuracy and reducing implantation failures.
- Canada is shaping up as a key player in mitochondrial diagnostics through academic-clinical partnerships and federal health initiatives promoting personalized reproductive care. Fertility centers are gradually implementing mtDNA assessment for embryo selection and maternal age-related risk prediction. Regulatory developments around MRT are being closely watched, and universities are contributing to the standardization of mtDNA biomarker-based testing within fertility workflows. Innovation hubs in Montreal and Toronto are actively funding biotech projects in reproductive genomics, while telehealth fertility platforms are exploring remote mtDNA test integration into pre-IVF consultations.
Asia-Pacific: Fastest-Growing Market
Asia-Pacific is emerging as the fastest-growing region for mtDNA fertility diagnostics, driven by rising IVF demand, rapid technological adoption, and growing awareness of mitochondrial health in reproductive care. Fertility clinics are increasingly using mtDNA content analysis for embryo scoring and age-related fertility risk mitigation. The region also fosters innovation in affordable testing solutions tailored for both urban and semi-urban settings. Government-backed reproductive health initiatives and growing investments in fertility biotech are accelerating access to mitochondrial diagnostics across diverse healthcare systems.
Additionally, increasing digital literacy, mobile health platforms, and startup-driven test delivery models are enabling faster market penetration and localized test customization.
- China’s market is expanding swiftly with the rise of IVF services and domestic biotechnology innovation. Government-funded institutes are focusing on mtDNA mutation analysis in reproductive disorders, with some pilot MRT programs launched in top-tier IVF clinics. There's a strong push toward in-country development of mtDNA test kits and AI-driven embryo analysis tools. Regulatory approval processes are tightening, yet supportive, aligning with China’s broader goal of healthcare self-reliance and reproductive technology advancement. Partnerships between public hospitals and biotech firms are facilitating clinical validation of mtDNA diagnostics, while government subsidies support local manufacturing and regional deployment in fertility hubs like Shanghai and Shenzhen.
- India’s rapidly growing fertility market is beginning to integrate mitochondrial diagnostics, especially among urban IVF networks and genomics-focused startups. Efforts are underway to introduce embryo scoring based on mtDNA content and track inheritance risks in older patients. Government bodies like ICMR are drafting MRT guidelines to align with international ethical standards, while national grants encourage academic research into mitochondrial fertility biomarkers. India’s growing digital health infrastructure supports early adoption in urban and semi-urban centers. Fertility education campaigns and insurance reforms are also accelerating consumer demand, with leading IVF chains piloting bundled packages that include mtDNA testing alongside standard PGT-A protocols.
Europe: Significant Growth
Europe is witnessing strong growth in mtDNA-based fertility diagnostics due to regulatory clarity, ethical frameworks, and integration into mainstream IVF workflows. Regional fertility centers are adopting mitochondrial assessments for embryo quality and donor egg screening, particularly in cross-border reproductive care settings. Biotech innovation is fueling the availability of standardized, lab-compatible mtDNA kits that align with stringent safety and data privacy standards. A well-established clinical research environment and emphasis on patient-centric diagnostics continue to support the region’s expanding role in this market.
Ongoing EU-funded reproductive health programs and public-private partnerships are boosting adoption across both public and private IVF networks, with sustainability and clinical transparency at the forefront.
- Germany is a key hub for mitochondrial fertility innovation in Europe, driven by its structured regulatory environment and advanced clinical research. Fertility clinics are piloting mtDNA testing protocols for donor egg selection and embryo health monitoring. With a strong focus on ethical compliance, German institutions emphasize validated technologies supported by EU frameworks. Government-backed initiatives are also working to expand accessibility to next-gen reproductive diagnostics under public healthcare systems. German biotech firms are investing in portable mtDNA assay platforms tailored for smaller fertility clinics, while patient advocacy groups promote awareness of mitochondrial conditions linked to reproductive health.
- The UK stands as a pioneer in legalizing MRT and integrating mtDNA testing into fertility protocols. Fertility centers and national research institutions are leading global efforts in sequencing mitochondrial genomes to prevent disease transmission and optimize IVF outcomes. Policy support from the HFEA and the NHS accelerates access to advanced diagnostics, positioning the UK as a benchmark market for regulated, ethically approved mitochondrial fertility care. Collaborative projects like the 100,000 Genomes Programme include fertility-related mtDNA markers, while NHS-funded pilot programs are testing the cost-effectiveness of integrating mtDNA testing into mainstream IVF cycles.
Market Segmentation
Product Type Insights
mtDNA-Based Fertility Testing Kits dominate this subsegment by enabling assessment of mitochondrial health in embryos, oocytes, and donor eggs. These kits typically include mtDNA quantification assays, long-range PCR tools, and targeted NGS panels tailored for fertility applications such as preimplantation genetic testing for aneuploidy (PGT-A) with mitochondrial scoring. Portable and low-input mtDNA kits designed for fresh and frozen embryo biopsies are gaining traction, particularly in time-sensitive fertility settings.
As the need for rapid embryo analysis grows, innovations in microfluidic mtDNA testing formats are poised to make procedures faster and more cost-effective.
Application Insights
Embryo Viability and Donor Egg Screening are the core applications in this subsegment. Clinics use mtDNA copy number analysis to predict embryo implantation potential, optimize embryo selection, and reduce miscarriage risks. In donor egg programs, mtDNA integrity is screened to ensure higher success rates and minimize transmission of mitochondrial abnormalities. Advanced applications include evaluating mitochondrial heteroplasmy post-MRT and monitoring oocyte rejuvenation therapies. These diagnostic insights allow reproductive specialists to tailor IVF protocols, improving patient outcomes and cost-efficiency.
Distribution Channel Insights
Fertility Clinics and IVF Centers serve as the primary distribution channels for mtDNA fertility testing. These centers either operate in-house genetics labs or collaborate with specialized diagnostic partners to process samples and interpret results. Increasingly, partnerships between kit manufacturers and assisted reproductive technology (ART) providers are enabling bundled solutions that integrate testing, analytics, and counseling into a single clinical workflow. Direct-to-clinic models and regional distributors are helping to penetrate emerging markets, particularly in Asia-Pacific and the Middle East, where IVF demand is rising. As precision fertility medicine becomes mainstream, distributors offering integrated support services are gaining a competitive edge.
End User Insights
Reproductive endocrinologists are the primary end users of mitochondrial DNA (mtDNA) testing in fertility medicine. They utilize mtDNA insights to enhance embryo selection, especially for patients with advanced maternal age, unexplained infertility, or repeated IVF failure. mtDNA-based embryo scoring helps these specialists identify embryos with higher implantation potential by assessing mitochondrial load, a key indicator of cellular viability. With the growing clinical adoption of mitochondrial replacement techniques and oocyte donation, reproductive endocrinologists are increasingly integrating mtDNA metrics into personalized stimulation protocols.
Company Market Share
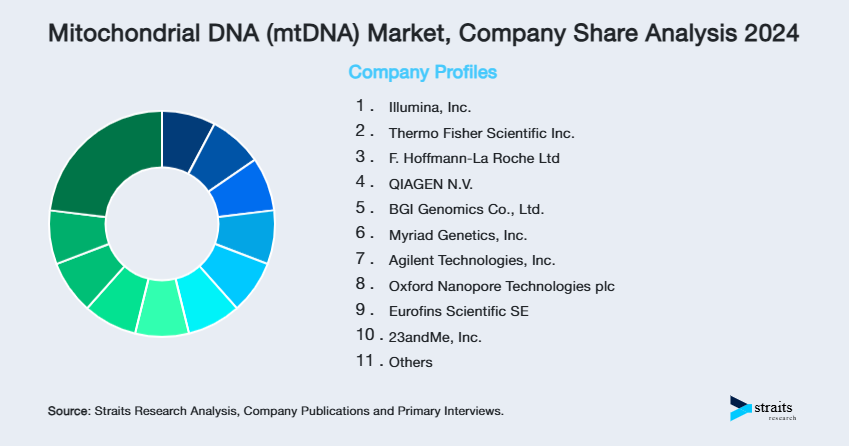
The mitochondrial DNA (mtDNA) market is moderately concentrated, with a handful of dominant players controlling a significant share of the global diagnostics and research solutions landscape. Company dominance is underpinned by proprietary sequencing platforms, robust bioinformatics capabilities, and expanding clinical partnerships across fertility clinics, ancestry services, and disease diagnostics.
Illumina, Inc. is a leading biotechnology company specializing in genomic sequencing and analysis technologies. Founded in 1998 and based in San Diego, it provides tools that accelerate research in precision medicine and genetic diseases. Illumina’s innovations drive advances in healthcare and life sciences worldwide.
- In April 2025, Illumina introduced enhanced mtDNA spike-in panels designed to integrate seamlessly with whole-exome sequencing workflows. These panels facilitate comprehensive mitochondrial analysis, including the detection of low-frequency variants and heteroplasmy, which are crucial for accurate genetic counseling and disease risk assessment.
List of Key and Emerging Players in Mitochondrial DNA (mtDNA) Market
- Illumina, Inc.
- Thermo Fisher Scientific Inc.
- F. Hoffmann-La Roche Ltd
- QIAGEN N.V.
- BGI Genomics Co., Ltd.
- Myriad Genetics, Inc.
- Agilent Technologies, Inc.
- Oxford Nanopore Technologies plc
- Eurofins Scientific SE
- 23andMe, Inc.
- FamilyTreeDNA (Gene by Gene, Ltd.)
- Invitae Corporation
- Genetic Technologies Limited
- Labcorp (Laboratory Corporation of America Holdings)
- Nebula Genomics, Inc.
to learn more about this report Download Market Share
Recent Developments
- May 2025- Researchers at Fujita Health University in Japan, led by Senior Assistant Professor Naoki Yahata, developed a novel enzyme technology using mitochondrial-targeted platinum transcription activator-like effector nucleases (mpTALENs) to selectively modulate mutant mitochondrial DNA (mtDNA) levels in patient-derived stem cells. This system enables precise editing of specific pathogenic mtDNA mutations by either increasing or decreasing mutant loads.
- May 2025- Khondrion, a biotech company focused on primary mitochondrial diseases (PMDs), secured up to €5 million in Innovation Credit from the Netherlands Enterprise Agency, matched by private investment. The funding will support a pivotal Phase 3 clinical trial of sonlicromanol, a first-in-class therapy targeting the m.3243A>G mitochondrial DNA mutation.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 324.8 million |
| Market Size in 2025 | USD 347.2 million |
| Market Size in 2033 | USD 612.6 million |
| CAGR | 7.2% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product Type, By Application, By Distribution Channel, By End-User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Mitochondrial DNA (mtDNA) Market Segments
By Product Type
- mtDNA-Based Fertility Testing Kits
- Next-Generation Sequencing (NGS)-Based Kits
- PCR-Based mtDNA Testing Kits
- Whole Mitochondrial Genome Panels
- Bioinformatics Analysis Software
By Application
- Embryo Viability and Donor Egg Screening
- Fertility and Reproductive Health Diagnostics
- Mitochondrial Disorder Screening
- Ancestry and Genealogy Research
- Cancer Biomarker Detection
- Neurodegenerative and Metabolic Disease Studies
By Distribution Channel
- Fertility Clinics and IVF Centers
- Hospitals and Diagnostic Laboratories
- Genetic Testing Companies (e.g., 23andMe, MyHeritage)
- Academic & Research Institutions
- Direct-to-Consumer (DTC) Platforms
- Government and Public Health Agencies
By End-User
- Reproductive endocrinologists
- Clinical Diagnostics Laboratories
- Pharmaceutical & Biotech Companies
- Academic & Genomic Research Centers
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Dhanashri Bhapakar
Senior Research Associate
Dhanashri Bhapakar is a Senior Research Associate with 3+ years of experience in the Biotechnology sector. She focuses on tracking innovation trends, R&D breakthroughs, and market opportunities within biopharmaceuticals and life sciences. Dhanashri’s deep industry knowledge enables her to provide precise, data-backed insights that help companies innovate and compete effectively in global biotech markets.










































