Next-Generation Gynecological Cancer Diagnostics Market Size, Share & Trends Analysis Report By Technology (Next generation sequencing, Polymerase chain reaction (PCR), DNA Microarrays, Multiplexing, Protein Microarrays, Others), By Cancer Type (Breast, Lung, Colorectal, Prostate, Cervical, Others), By Function (Cancer Screening, Therapeutic Monitoring, Risk Analysis) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Next-Generation Gynecological Cancer Diagnostics Market Overview
The global next-generation cancer diagnostics market size is estimated at USD 17.98 billion in 2025 and is projected to reach USD 45.61 billion by 2034, growing at a CAGR of 10.94% during the forecast period. Strong growth of the market is propelled by the growing government and private initiatives aimed at improving cancer diagnosis and care.
Key Market Trends & Insights
- According to Straits Research, North America held a dominant share of the global market, accounting for 40.93% in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 13.01%.
- Based on Technology, the next-generation sequencing segment dominated the market in 2025 with a revenue share of 36.74%.
- Based on Type, the breast cancer segment is expected to register the fastest CAGR growth of 11.70%.
- Based on Function, the cancer screening segment dominated the market in 2025, with a revenue share of 45.23%.
- The U.S. dominates the global next-generation cancer diagnostics market, valued at USD 6.14 billion in 2024 and reaching USD 6.78 billion in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
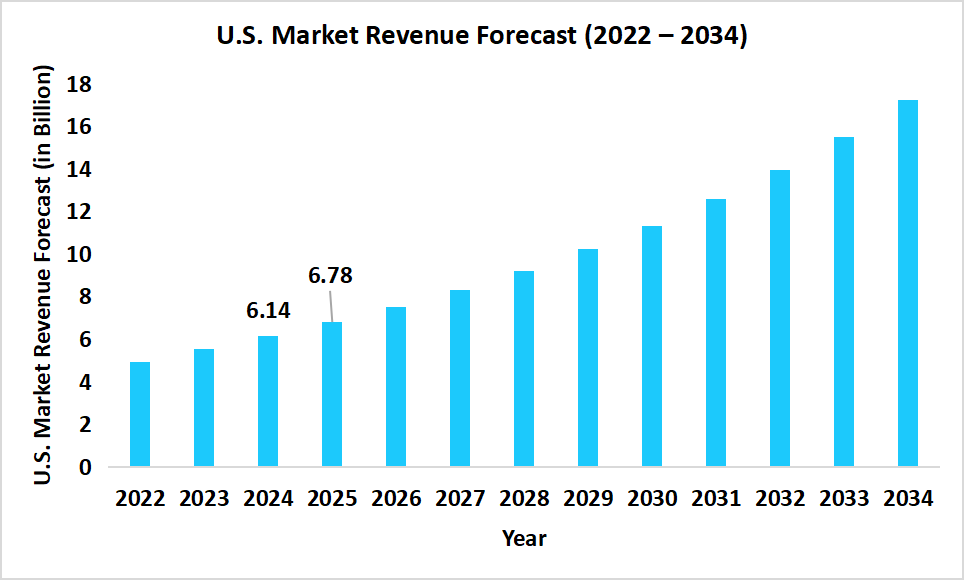
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 17.98 billion
- 2034 Projected Market Size: USD 45.61 billion
- CAGR (2025 to 2034): 10.94%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The global next-generation cancer diagnostics market encompasses a range of technologies, including next-generation sequencing, polymerase chain reaction (PCR), DNA microarrays, multiplexing, protein microarrays, and other emerging platforms, catering to the detection and analysis of various cancer types. Key cancer indications addressed by these diagnostics include breast, lung, colorectal, prostate, cervical, and other cancers, enabling early detection, personalized treatment planning, and monitoring of disease progression. These diagnostics serve multiple functional purposes, such as cancer screening, therapeutic monitoring, and risk analysis, allowing healthcare providers to implement precision oncology approaches. Adoption of these technologies is influenced by factors such as increasing cancer prevalence, the rising demand for minimally invasive testing, integration of bioinformatics and AI tools, and supportive regulatory and reimbursement frameworks across global healthcare systems.
Latest Market Trends
Shift from Invasive to Non-Invasive Cancer Screening
A major trend in the next-generation cancer diagnostics market is the shift from invasive procedures to non-invasive blood-based tests for early cancer detection. Recently, Guardant Health received FDA approval for its Shield test, a blood-based diagnostic that detects somatic mutations and epigenomic changes in cell-free DNA (cfDNA) associated with colorectal cancer.
This indicated that this transition facilitated earlier and more accessible cancer detection, reduced the demand for invasive procedures, and supported timely clinical decision making.
AI-Driven Digital Pathology: A Major Trend in Next-Generation Cancer Diagnostics
A key trend in the next-generation cancer diagnostics market is the advancement of AI powered digital pathology, which enhances the accuracy and efficiency of cancer diagnosis. Several companies are investing in AI-driven digital pathology. Notably, F. Hoffmann-La Roche Ltd expanded its Digital Pathology Open Environment by integrating over 20 advanced AI algorithms from eight new collaborators. These tools were accessible through Roche’s Navify Digital Pathology platform, which supported pathologists in analysing tissue samples more precisely, and also facilitated personalized treatment plans for cancer patients.
This highlighted that the integration of AI-powered tools in digital pathology and transformed cancer diagnostics by streamlining tissue analysis and enhancing the ability of clinicians to make informed, patient-specific treatment decisions.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 17.98 Billion |
| Estimated 2026 Value | USD 19.88 Billion |
| Projected 2034 Value | USD 45.61 Billion |
| CAGR (2026-2034) | 10.94% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Johnson & Johnson, Illumina, Inc., Novartis AG, Hoffmann-La Roche Ltd, Koninklijke Philips N.V. |

to learn more about this report Download Free Sample Report
Next-Generation Gynecological Cancer Diagnostics Market Driver
Automated Multiplex Imaging Drives Advancements in Cancer Diagnostics
A key driving factor in the next-generation cancer diagnostics market is the creation of automated multiplex tools that makes analysis of complex tissue samples easier. For example, in September 2025, scientists of Mount Sinai launched the MARQO pipeline, an automated system that analysed complex cancer tissues at single cell level using advanced staining techniques. It enabled accurate measurement of immune and tumor cells in lung, colorectal, melanoma and liver cancer samples.
Such a factor depicted the development of automated multiplex tools, which enhanced the efficiency and accuracy of cancer tissue analysis, supported more precise diagnostics, and informed treatment decisions across multiple cancer types.
Market Restraint
Limited and Fragmented Reimbursement for NGS Testing
A major restraint for the next-generation cancer diagnostics market is the fragmented and restricted reimbursement landscape, particularly in the Asia Pacific region. The Asia Pacific Medical Technology Association (APACMed) highlighted that reimbursement for NGS testing is often limited to specific tests, panel types, or cancer types, such as non-small cell lung cancer (NSCLC), in certain jurisdictions. This fragmented reimbursement led to inequities in NGS access, leaving other populations who may benefit from the technology without coverage.
Market Opportunity
Transforming Cancer Care with Next Generation Multi-Cancer Early Detection (MCED)
An opportunity for the next generation cancer diagnostics market lies in the development of non-invasive MCED tests capable of detecting multiple cancers at the earliest stages using simple samples such as breath, urine, or blood. Programs, such as ARPA-H’s POSEIDON, launched to accelerate advanced diagnostic solutions, provided manufacturers with opportunities to commercialize disruptive technologies.
By leveraging these initiatives, companies expanded their global reach, enhanced oncology diagnostics portfolios, and contributed to improved survival rates through faster and more accessible cancer detection.
Regional Analysis
The North America region dominated the market with a revenue share of 40.93% in 2025. The growth is attributed to factors such as widespread adoption of liquid biopsy NGS tests for early cancer detection which improved diagnostic accuracy, enabled minimally invasive testing, and enhanced higher clinical acceptance across oncology practices, further strengthening the region’s leadership in next-generation cancer diagnostics.
The next-generation cancer diagnostics market in U.S. is widely driven by Medicare’s coverage of FDA-approved NGS tests for advanced cancer. This policy ensured that patients get reimbursed for next generation sequencing tests, which hence made the tests more affordable. This unique step expanded access, increased adoption, and boosted market size, as well as strongly supported overall next-generation cancer diagnostics market growth.
Asia Pacific Market Insights
The Asia Pacific region is the fastest-growing region with a CAGR of 13.01% during the forecast timeframe. The growth is attributed to factors such as government funding for cancer genomics research in Japan and South Korea which has accelerated advancements clinical adoption, and fostered collaborations between research institutions and industry players, while also creating opportunities for early detection programs, and expansion of next generation diagnostic technologies across emerging economies in the region.
India continues to position itself in the next-generation cancer diagnostics market, due to the low-cost access to advanced NGS tests for lung cancer. In 2024, AstraZeneca India and RGCI&RC jointly launched a dedicated center that offered a 34-gene panel NGS test at subsidized rates. This initiative not only improved patient access to precision diagnostics but also enhanced awareness, which further contributed to expanding the overall market growth in the country.
Pie Chart: Regional Market Share, 2025
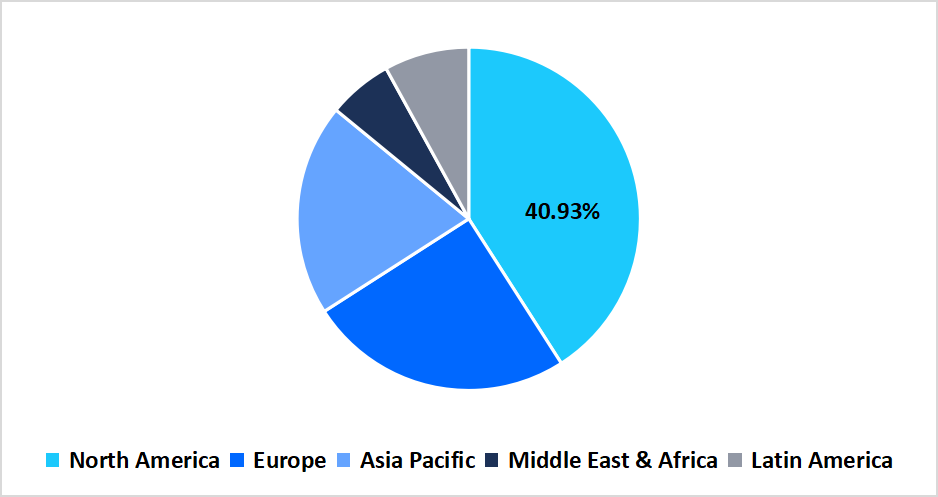
Source: Straits Research
Europe Market Insights
A major driving factor for the European next-generation cancer diagnostics market is the region’s rapid digitalization of healthcare and integration of artificial intelligence (AI) in diagnostic workflows. The implementation of the Digital Healthcare Act (DVG) and growing investments in AI-enabled pathology and imaging platforms are transforming cancer diagnostics into a more data-driven and precise process. Hospitals and diagnostic centers are increasingly adopting digital pathology systems and cloud-based genomic analysis tools that enable faster and more accurate interpretation of tumor profiles.
The next-generation cancer diagnostics market growth is propelling due to the research and development in this field, which is further accelerating market growth, with companies heavily investing in R&D and leveraging government funding to advance oncology diagnostics. This strong focus on development is driving higher adoption of NGS-based cancer diagnostics, further contributing to overall market expansion. The trend is clearly reflected in the rising investment patterns, as depicted in the graph below, which illustrates Cancer Research UK’s spending on ongoing and new cancer research in 2023:
Graph: Spending on Ongoing & New Cancer Research by Cancer Research UK, 2023
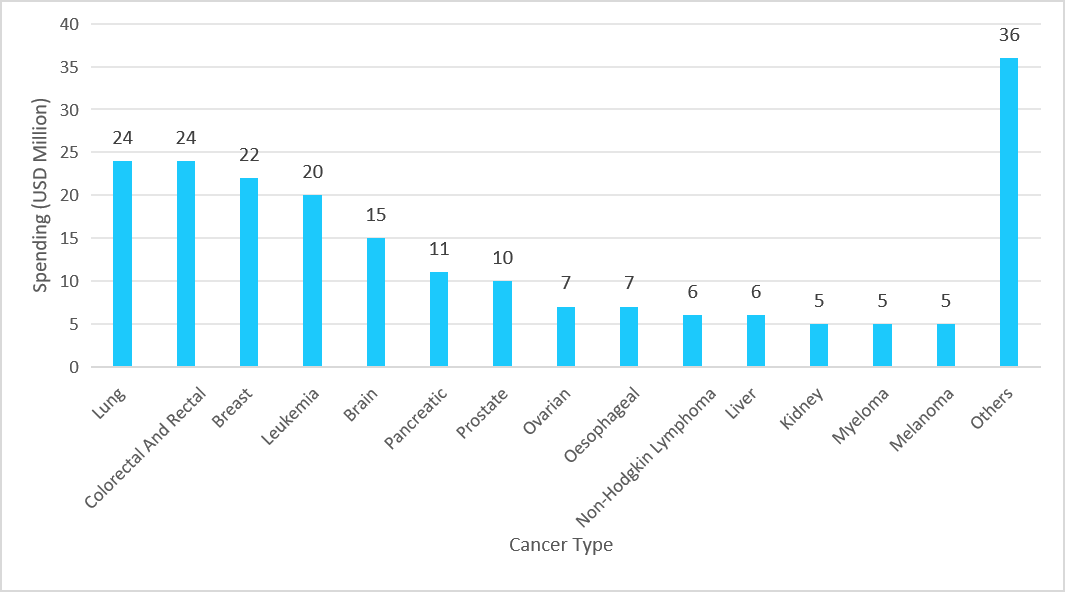
Source: Straits Research
Middle East and Africa Market Insights
In the Middle East and Africa, a unique driving factor for the next-generation cancer diagnostics market is the rising demand for non-invasive and rapid diagnostic solutions due to limited access to advanced oncology facilities in several regions. Several countries are adopting portable molecular testing technologies to overcome challenges associated with traditional tissue biopsies, such as the lack of specialized laboratories and pathologists. For instance, the United Arab Emirates and South Africa are introducing point-of-care genomic testing initiatives to expand cancer screening and early detection in remote areas.
In South Africa, a distinctive driving factor for the next-generation cancer diagnostics market is the increasing integration of oncology diagnostics within public and private research collaborations aimed at addressing the country’s diverse genetic landscape. South Africa’s population exhibits genetic heterogeneity, which has prompted local research institutions to prioritize genomic studies tailored to African populations. Initiatives such as the South African National Bioinformatics Institute (SANBI) and collaborations with the South African Medical Research Council (SAMRC) are supporting the development of region-specific cancer biomarkers and molecular diagnostic assays.
Latin America Market Insights
In Latin America, a key driving factor for the next-generation cancer diagnostics market is the expansion of regional manufacturing and localization of diagnostic technologies to reduce dependency on imports and improve affordability. Several countries, including Brazil, Mexico, and Argentina, promoted domestic production of molecular testing reagents, sequencing kits, and diagnostic instruments through public-private partnerships and government-backed funds. For instance, Brazil’s Ministry of Health has supported initiatives under the Productive Development Partnerships (PDPs) program to enhance local manufacturing of precision diagnostic tools, including next-generation sequencing (NGS) assays for oncology applications.
In Mexico, the driving factor for the next-generation cancer diagnostics market is the increasing focus on population-specific biomarker research to address regional cancer prevalence patterns. Mexican research institutions and universities conducted studies to identify genetic mutations and molecular markers that are more prevalent in Latin American populations, enabling the development of more accurate and tailored diagnostic tests. For example, collaborations between the National Institute of Genomic Medicine (INMEGEN) and private biotech firms led to the creation of targeted NGS panels for breast, cervical, and gastric cancers, which are among the most common in the region. This emphasis on locally relevant genomic data boosted the adoption of advanced cancer diagnostic technologies across Mexico.
Technology Insights
The next-generation sequencing (NGS) segment dominated the market in 2025 with a revenue share of 36.74%, due to the increasing adoption of multi-gene panel testing and personalized treatment planning, coupled with advancements in high-throughput sequencing technologies, greater clinical accuracy, and growing awareness among healthcare providers and patients about the benefits of precision oncology.
Polymerase chain reaction (PCR) is anticipated to register the fastest CAGR of 11.34%, during the forecast period, owing to its high sensitivity and specificity in detecting low levels of tumor DNA, RNA, or specific mutations, which is crucial for early cancer detection and monitoring minimal residual disease.
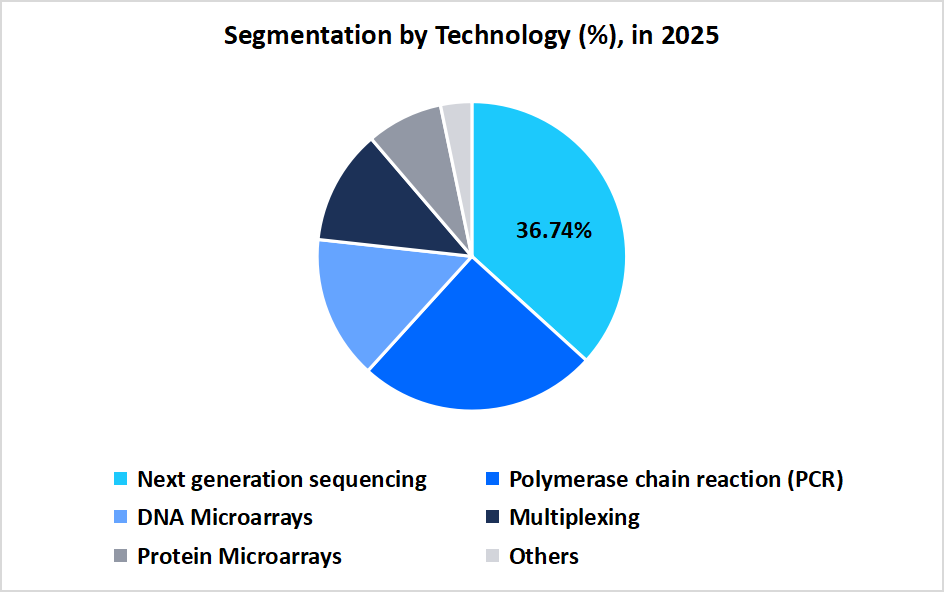
Source: Straits Research
Type Insights
The breast cancer segment is anticipated to grow at a CAGR of 11.70% during 2026-2034, owing to the rising awareness of hereditary breast cancer and the focus on development of targeted therapies for its treatment.
The lung cancer segment dominated the market in 2025, with a revenue share of 46.24%, owing to growing incidence of smoking-related and environmental risk factors, advancements in minimally invasive diagnostic techniques, and the rising availability of companion diagnostic tests. Increased investment in research for novel biomarkers and targeted therapies has also accelerated the adoption of next-generation diagnostics.
Function Insights
The cancer screening dominated the market in 2025, with a revenue share of 45.23%. The growth is attributed to the rising cancer prevalence and strong government initiatives promoting early detection programs. Moreover, the adoption of advanced diagnostics is driving the cancer screening segment’s growth by improving outcomes and enabling timely treatment interventions.
The therapeutic monitoring segment is expected to register the fastest CAGR of 11.21% during the forecast period, owing to the increasing adoption of targeted therapies and immunotherapies in cancer treatment, which has further enhanced the demand for efficient therapeutic monitoring. These advanced treatment modalities often require close monitoring to evaluate their impact on the tumor and ensure optimal patient outcomes.
Competitive Landscape
The global next-generation cancer diagnostics market is highly fragmented, with numerous players focusing on niche technologies, regional markets, and specialized diagnostic solutions.
Burning Rock Dx: An emerging market player
Burning Rock Dx, is an emerging player in the next-generation cancer diagnostics market, specializing in precision oncology through NGS-based diagnostics, companion diagnostics development, and early cancer detection.
- In October 2024, Burning Rock Dx announced that its OncoCompass Focus CDx test, which detected 9 major gene mutations, and was approved by China’s National Medical Products Administration (NMPA). The test was used as a companion diagnostic with Dizal’s targeted therapy, sunvozertinib for patients with EGFR exon 20 insertion mutations.
List of Key and Emerging Players in Next-Generation Gynecological Cancer Diagnostics Market
- Johnson & Johnson
- Illumina, Inc.
- Novartis AG
- Hoffmann-La Roche Ltd
- Koninklijke Philips N.V.
- QIAGEN
- Agilent Technologies, Inc.
- Abbott
- Thermo Fisher Scientific Inc.
- GE HealthCare
- Karkinos Healthcare
- GenScript
- Exact Sciences Corporation
- Bio-Rad Laboratories, Inc.
- Almac Group
- Cepheid
- Natera, Inc.
- Hologic, Inc.
- Sysmex Corporation
- PerkinElmer Life Sciences & Diagnostics
- Others
Strategic Initiatives
- June 2025: QIAGEN and Incyte announced a global collaboration to develop NGS based companion diagnostic panel for mutant CALR driven myeloproliferative neoplasms.
- June 2025: Gene Solutions partnered with ZaoDx and Xiong'an MagicBiotech Co., Ltd., which signalled a strategic expansion of its precision oncology initiatives in the Chinese market by introducing advanced technologies, fostering research collaborations, and deploying cancer testing platforms locally.
- May 2025: Illumina, Inc. expanded its oncology portfolio with the TruSight Oncology Comprehensive test, the first FDA approved distributable genomic profiling kit with pan cancer companion diagnostic claims.
- January 2025: Guardant Health announced the expansion of Guardant Reveal MRD test into the additional solid tumor indications, which strengthened its liquid biopsy portfolio for recurrence monitoring.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 17.98 Billion |
| Market Size in 2026 | USD 19.88 Billion |
| Market Size in 2034 | USD 45.61 Billion |
| CAGR | 10.94% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Technology, By Cancer Type, By Function |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Next-Generation Gynecological Cancer Diagnostics Market Segments
By Technology
- Next generation sequencing
- Polymerase chain reaction (PCR)
- DNA Microarrays
- Multiplexing
- Protein Microarrays
- Others
By Cancer Type
- Breast
- Lung
- Colorectal
- Prostate
- Cervical
- Others
By Function
- Cancer Screening
- Therapeutic Monitoring
- Risk Analysis
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































