NUT Midline Carcinoma Treatment Market Size, Share & Trends Analysis Report By Treatment (Immunotherapy, Targeted Therapy, Chemotherapy, Radiation Therapy, Others), By Route Of Administration (Oral, Intravenous, Other), By End User (Hospitals, Specialty Clinics, Other) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
NUT Midline Carcinoma Treatment Market Overview
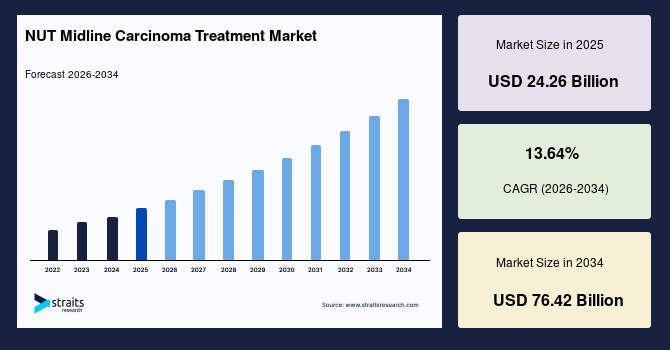
The global NUT midline carcinoma treatment market size is valued at USD 24.26 billion in 2025 and is estimated to reach USD 76.42 billion by 2034, growing at a CAGR of 13.64% during the forecast period. The accelerated growth of the market is driven by the integration of AI‑guided epigenetic profiling, allowing ultra personalized NMC therapy selection.
Key Market Trends & Insights
- North America held a dominant revenue share of the global market, accounting for 39.43% in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 15.02% during the forecast period.
- Based on treatment, the chemotherapy segment dominated the market in 2025, accounting for 32.10% revenue share.
- Based on route of administration, the oral segment is estimated to grow at the fastest pace with a CAGR of 14.13% during the forecast period.
- Based on end user, the hospitals segment dominated the market in 2025 with a revenue share of 63.91%.
- The U.S. dominates the market, valued at USD 7.64 billion in 2024 and reaching USD 8.65 billion in 2025.
Table: U.S. NUT Midline Carcinoma Treatment Market Size (USD Million)
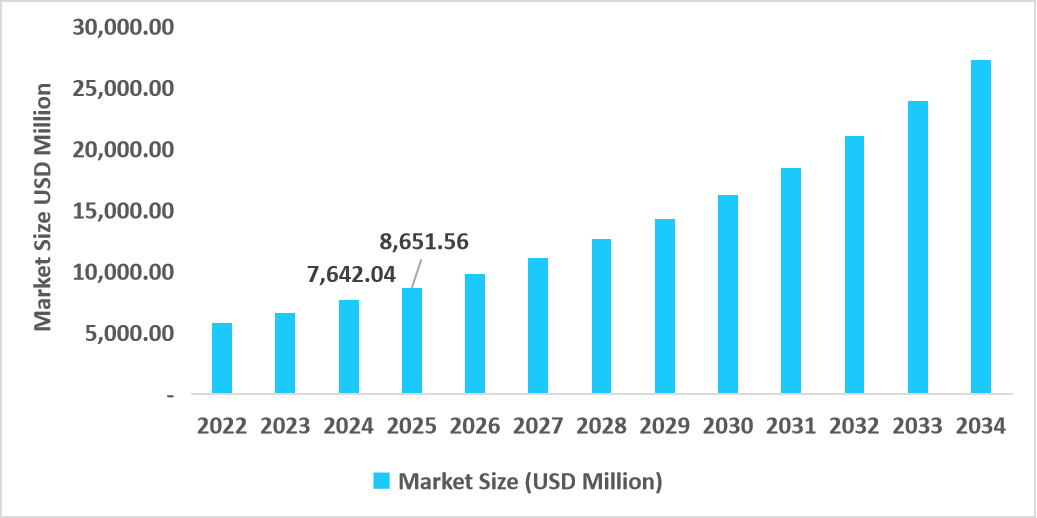
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 24.26 billion
- 2034 Projected Market Size: USD 76.42 billion
- CAGR (2026-2034): 13.64%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The NUT midline carcinoma treatment market encompasses therapies designed to manage and treat this rare and aggressive cancer. Key treatment modalities include immunotherapy, targeted therapy, chemotherapy, radiation therapy, and other emerging approaches. These therapies are administered via oral, intravenous, or alternative routes. The market serves hospitals, specialty clinics, and other healthcare settings, enabling providers to deliver precise, effective, and personalized cancer care.
Latest Market Trends
Rise of Precision Diagnostics in Nut Midline Carcinoma Treatment
A notable trend in the NUT midline carcinoma treatment market is the increasing use of advanced molecular diagnostics combined with artificial intelligence to improve the detection of rare NMC cases. Techniques such as RNA fusion testing, immunohistochemistry, and fluorescence in situ hybridization are becoming standard practice. Machine-learning tools applied to genomic and pathology data enhance diagnostic accuracy, uncover previously undiagnosed cases, and support more effective personalized therapies, driving market growth.
Growing Focus on Epigenetic Targeted Therapies and Combination Treatments
A key emerging trend in the market is the accelerated development and clinical exploration of epigenetic targeted therapies, particularly inhibitors that target the fusion-driven chromatin dysregulation underlying NMC. Combining BET inhibitors with other epigenetic modulators has shown strong preclinical synergy, effectively blocking cancer cell growth and reactivating tumor suppressor genes. This shift toward precise, mechanism-based combination therapies is driving innovation and offering patients more effective and less toxic treatment options.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 24.26 Billion |
| Estimated 2026 Value | USD 27.48 Billion |
| Projected 2034 Value | USD 76.42 Billion |
| CAGR (2026-2034) | 13.64% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Merck & Co., Inc., Bristol-Myers Squibb Company, Pfizer Inc., Hoffmann-La Roche Ltd, C4 Therapeutics, Inc. |
to learn more about this report Download Free Sample Report
Latest Market Drivers
Advancements in Genomic Diagnostics and Precision Medicine
The rapid improvement in genomic and molecular diagnostic technologies enables more accurate detection of NUTM1 gene rearrangements is a key driver supporting the market growth. These advancements allow clinicians to identify NMC cases earlier and more reliably, expanding the identifiable patient population. As a result, demand grows for precision therapies, including targeted agents such as BET inhibitors. This trend enhances treatment uptake and fuels market expansion.
Market Restraints
Extremely Limited Patient Population and High Cost of Treatment for nut Midline Carcinoma
A major restraint of the NUT midline carcinoma treatment market is the disease’s ultra rare incidence, coupled with the high cost of targeted treatments and diagnostics. Since only a few hundred NMC cases have been reported worldwide, patient scarcity makes it difficult to conduct large-scale clinical trials, undermining evidence generation and slowing regulatory approvals. Moreover, advanced molecular diagnostics and novel therapies remain expensive, limiting access in lower income regions.
Market Opportunity
Expansion of Targeted Therapies & Regulatory Support for Rare Cancer Drugs
A major opportunity in the NUT midline carcinoma treatment market lies in the accelerating development of novel targeted therapies, such as agents like BET inhibitors aimed at the genetic drivers of NMC. As these therapies advance through clinical trials, they provide hope for more effective and personalized treatment options beyond conventional chemotherapy. Coupled with favorable regulatory incentives, this creates a favorable environment for pharmaceutical companies to invest in new drugs. As a result, the market is poised to benefit from growing treatment uptake, improved patient outcomes, and expanding therapeutic options for this rare cancer.
Regional Analysis
North America dominated the market in 2025, accounting for 39.43% market share. Growth in the region is supported by the extensive establishment of rare cancer research networks and patient registries. These systems enable faster diagnosis, facilitate clinical trial enrollment, and enhance access to innovative therapies, supporting higher treatment adoption and accelerating market growth across the region.
The U.S. NUT midline carcinoma treatment market is experiencing unique growth driven by the FDA’s strong support for orphan drugs under the Orphan Drug Act. Incentives such as tax credits, fee waivers, and seven-year marketing exclusivity encourage pharmaceutical investment in rare-cancer therapies, boosting development and market growth.
Asia Pacific Market Insights
Asia Pacific is emerging as the fastest growing region with a CAGR of 15.02% from 2026-2034. The growth is attributed to rising volume of oncology focused clinical trials leveraging large patient populations across China, India, South Korea, and Southeast Asia. This accelerated trial activity expands the evidence base for rare cancer therapies, increases drug availability, and speeds up regulatory approvals, boosting overall treatment adoption and market growth.
Australia NUT midline carcinoma treatment market is experiencing strong growth during the forecast period. This growth is attributed to the expansion of the Australian Rare Cancer Portal. This platform connects patients with expert oncologists, facilitates genomic profiling, and improves access to clinical trials, enhancing treatment availability and supporting market growth nationwide.
Regional Market share (%) in 2025
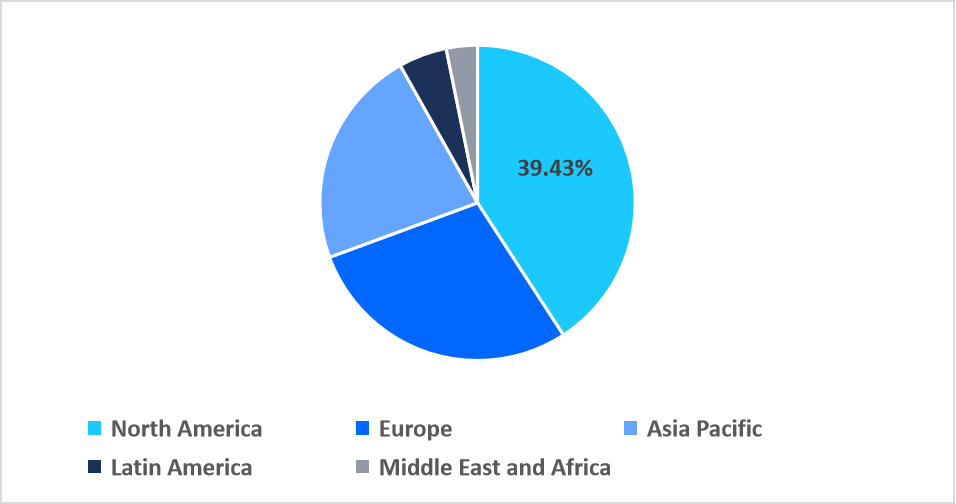
Source: Straits Research
Europe Market Insights
Europe is witnessing strong growth in the market, driven by the development of cross‑border rare cancer networks, such as EURACAN. These networks connect specialized centers, facilitate shared expertise, coordinated clinical trials, and improve patient access to advanced therapies, supporting market growth across the region.
Germany NUT midline carcinoma treatment market is stimulated by the launch of a national plan for gene and cell based therapies, coordinated through the multi site National Center for Tumor Diseases (NCT). This initiative, backed by the federal government, fast‑tracks innovative molecular treatments for rare cancers and improves clinical trial access nationwide, which, in turn, contributes to the market growth.
Latin America Market Insights
The Latin America market is benefiting from the increasing adoption of next-generation sequencing oncology diagnostics. Enhanced detection of rare cancers in countries like Brazil and Mexico improves access to precision therapies, supporting treatment uptake and overall market growth.
The Brazil NUT midline carcinoma treatment market is propelled by the country’s emergence as a hub for global oncology clinical trials. Its large, genetically diverse population and streamlined regulatory pathways enhance rare cancer study enrollment, improving patient access to innovative therapies and supporting market growth.
Middle East and Africa Market Insights
The Middle East and Africa market is being driven by the rapid development of genomics-enabled oncology centers in countries like the UAE and Saudi Arabia, integrating next-generation sequencing and molecular diagnostics to enhance rare cancer detection and improve patient access to advanced therapies.
The Saudi Arabia NUT midline carcinoma treatment market is benefiting from the rising integration of genomic medicine infrastructure through centers such as King Faisal Specialist Hospital & Research Centre (KFSHRC), which offers in-house gene sequencing, CAR‑T manufacturing, and precision oncology services, which allow faster rare cancer diagnosis and access to advanced therapies.
Treatment Insights
The chemotherapy segment dominated the market with a revenue share of 32.10% in 2025. This dominance is attributed to the development of nanoparticle encapsulated cytotoxic drugs specifically targeting NUT fusion-positive cells, which enhances drug delivery precision, minimizes systemic toxicity, and improves therapeutic outcomes for patients resistant to conventional chemotherapy.
The immunotherapy segment is projected to grow at a fastest CAGR of 13.98% during 2026-2034, owing to the use of personalized neoantigen vaccines targeting unique NUT fusion derived antigens, which stimulate patient specific immune responses, enhancing efficacy and reducing off-target effects compared to conventional immunotherapy approaches.
By Treatment Market Share (%), 2025
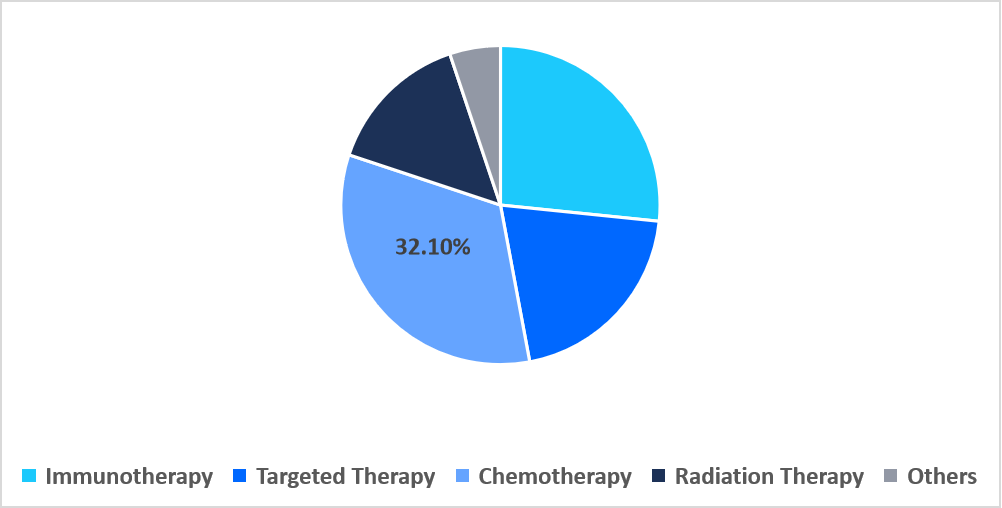
Source: Straits Research
Route of Administration Insights
The intravenous segment dominated the market with a revenue share of 67.14% in 2025. This dominance is augmented by the adoption of continuous infusion microfluidic pumps, which allow precise, controlled delivery of NUT midline carcinoma therapies, improving drug bioavailability and reducing systemic toxicity compared to standard bolus injections.
The oral segment is anticipated to register the fastest CAGR of 14.13% during 2026-2034. This strong growth is driven by the development of pH-responsive, targeted polymeric capsules that protect NMC targeted drugs through the gastrointestinal tract, ensuring controlled release directly into systemic circulation, enhancing efficacy and patient adherence in long-term therapy.
End User Insights
The hospitals segment dominated the market with a revenue share of 63.91% in 2025. A key factor driving the segment growth is the integration of multidisciplinary NMC care units, combining oncology, genomics, and precision therapy teams under one roof, which enhances treatment coordination, improves patient outcomes, and reinforces hospitals as primary centers for advanced NMC management.
The specialty clinics segment is expected to register fastest CAGR growth during the forecast timeframe, due to their focus on outpatient, patient-centric infusion services for NMC therapies, offering flexible scheduling, personalized monitoring, and streamlined follow-ups, which attract patients preferring convenient, high-quality care outside traditional hospital settings.
Competitive Landscape
The NUT midline carcinoma treatment market is highly consolidated, with a few leading pharmaceutical and biotech companies holding a notable share. Key players include Merck & Co., Bristol‑Myers Squibb, Pfizer, Roche, C4 Therapeutics, Ipsen, and GSK, which focus on advancing targeted therapies and immunotherapies for NMC. These companies leverage strong R&D capabilities, strategic collaborations, and global distribution networks, while smaller biotech firms contribute to innovation in diagnostics and early-stage drug development, collectively shaping the competitive landscape.
Zenith Epigenetics: An emerging market player
Zenith Epigenetics is emerging as a noteworthy player in the market, focusing on novel BET inhibitor therapies for cancers driven by BRD‑NUT fusions. Its oral candidate ZEN‑3694 recently secured FDA fast‑track designation for metastatic or unresectable NUT midline carcinoma, underscoring the company’s growing clinical relevance and potential to expand therapeutic options in this rare‑cancer segment.
List of Key and Emerging Players in NUT Midline Carcinoma Treatment Market
- Merck & Co., Inc.
- Bristol-Myers Squibb Company
- Pfizer Inc.
- Hoffmann-La Roche Ltd
- C4 Therapeutics, Inc.
- Ipsen Biopharmaceuticals, Inc.
- GSK plc
- Others
- By Treatment
- Immunotherapy
- Targeted Therapy
- Chemotherapy
- Radiation Therapy
- Others
- By Route Of Administration
- Oral
- Intravenous
- Other
- By End User
- Hospitals
- Specialty Clinics
- Other
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 24.26 Billion |
| Market Size in 2026 | USD 27.48 Billion |
| Market Size in 2034 | USD 76.42 Billion |
| CAGR | 13.64% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Treatment, By Route Of Administration, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
NUT Midline Carcinoma Treatment Market Segments
By Treatment
- Immunotherapy
- Targeted Therapy
- Chemotherapy
- Radiation Therapy
- Others
By Route Of Administration
- Oral
- Intravenous
- Other
By End User
- Hospitals
- Specialty Clinics
- Other
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































