Nutraceutical Contract Manufacturing Services Market Size, Share & Trends Analysis Report By Formulations (Tablets, Capsules, Liquid, Gummies, Energy Bars, Other Suitable Forms), By Product (Dietary Supplements, Functional Food and Beverages) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Nutraceutical Contract Manufacturing Services Market Size
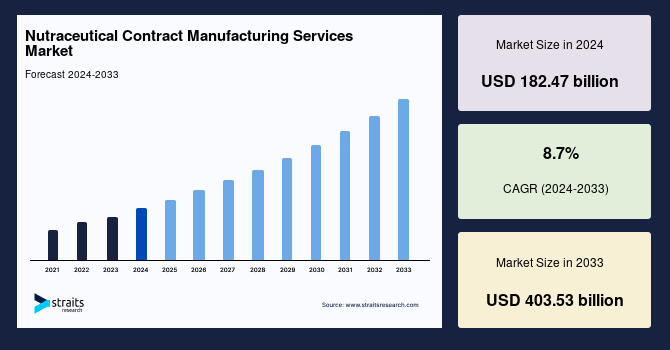
The global nutraceutical contract manufacturing services market size was valued at USD 182.47 billion in 2024 and is projected to grow from USD 198.34 billion in 2025 to reach USD 386.6 billion in 2033, growing at a CAGR of 8.7% during the forecast period (2025–2033).
Nutraceutical contract manufacturing services refer to outsourcing the production of nutraceutical products (such as dietary supplements, functional foods, and beverages) to a third-party manufacturer. These services allow companies to focus on other aspects of their business, like marketing and distribution, while leaving the production process to experienced manufacturers.
The contract manufacturer handles the entire production process, including sourcing raw materials, formulation, production, packaging, and labeling. This enables companies to create high-quality nutraceutical products without having to invest in manufacturing facilities or specialized equipment. The growing demand for dietary supplements is fueling innovation and research into novel nutraceutical formulations, accelerating the need for outsourcing in the nutraceutical market.
Moreover, the increasing prevalence of lifestyle-related diseases, which require effective dietary supplementation for management and prevention, is driving a surge in demand for nutraceutical products. This convergence of rising consumer awareness, health concerns, and scientific advancements in formulation is further propelling the growth of nutraceutical contract manufacturing services, making it a vital component of the industry.
The below chart shows 3 years (2020-2022) prevalence of the most common lifestyle diseases
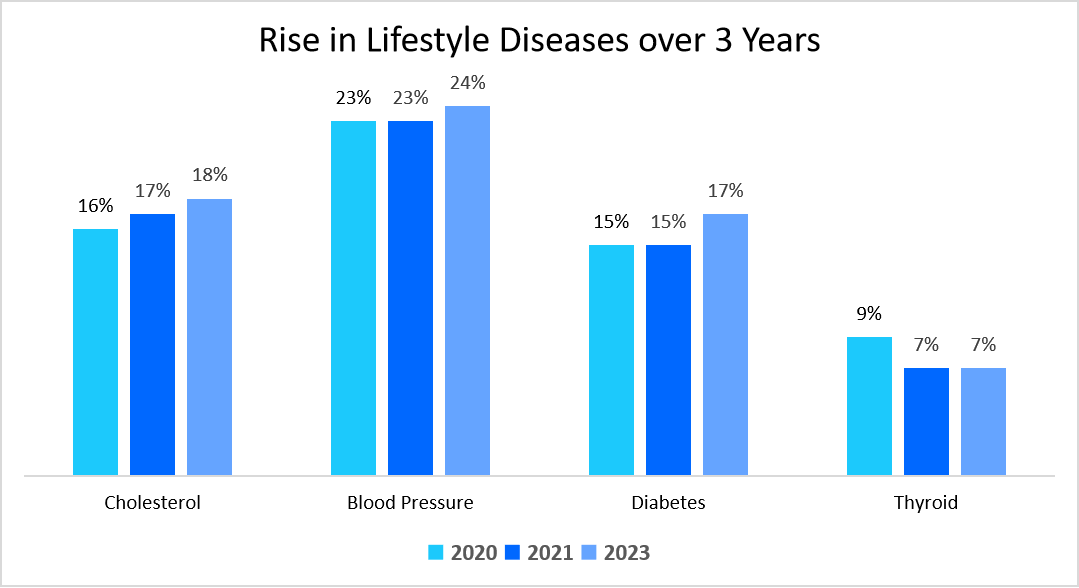
Source: Straits Research, GOQii
As per the data presented, the prevalence of diabetes is projected to rise from 15% in 2022 to 17% in 2023, underscoring the growing demand for natural solutions to manage the condition. As consumers increasingly seek effective, plant-based alternatives for diabetes care, the need for high-quality nutraceutical products intensifies.
Moreover, stringent quality standards and certifications such as cGMP and NSF further emphasize the importance of specialized contract manufacturers. These manufacturers ensure adherence to these rigorous requirements, enabling nutraceutical companies to scale efficiently and meet market demands.
Market Trends
Personalized Nutrition and Custom Formulations
The growing demand for personalized nutrition is driving the need for custom-formulated nutraceuticals tailored to specific health requirements, such as improving gut health, cognitive function, and boosting immune support. To meet this demand, contract manufacturers are increasingly adopting AI-driven formulation processes and small-batch production models, enabling them to create unique, targeted nutraceutical solutions.
- For example, a December 2023 study published in New Hope Network highlighted how AI is revolutionizing the development of customized food and nutrition formulations by utilizing vast data sets to predict individual health needs and optimize nutrient delivery.
This growing trend toward personalized nutraceuticals presents significant opportunities for specialized manufacturing services, thus propelling the market for contract manufacturing in the nutraceutical industry.
Expansion of Plant-Based Manufacturing
Consumer demand for vegan, organic, and minimally processed nutraceuticals is reshaping the nutraceutical market, compelling contract manufacturers to adapt their production processes to meet these evolving preferences. As more consumers seek plant-based alternatives for better health and sustainability, manufacturers are incorporating advanced techniques to preserve nutrients and maintain product quality.
- For instance, Zeon Lifesciences Ltd. reported in September 2024 that liposomal contract manufacturing is gaining momentum within plant-based nutraceutical production. This method enhances nutrient absorption and improves product efficacy, appealing to the growing plant-based consumer base.
As plant-based nutraceutical products continue to grow in popularity, the need for specialized contract manufacturing services is increasing.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 182.47 Billion |
| Estimated 2025 Value | USD 198.34 Billion |
| Projected 2033 Value | USD 386.6 Billion |
| CAGR (2025-2033) | 8.7% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Ashland, Glanbia PLC, Herbalife International of America, Inc., Biotrex Nutraceuticals, Martínez Nieto |
to learn more about this report Download Free Sample Report
Nutraceutical Contract Manufacturing Services Market Growth Factors
Regulatory Compliance and Quality Control
Governments and health authorities, such as the FDA in the U.S., EFSA in Europe, and other national regulatory bodies worldwide, are imposing stricter guidelines for consumer safety, product efficacy, and label transparency. These regulations cover every aspect of production, from sourcing ingredients to manufacturing processes, packaging, and labeling, all of which can vary significantly across regions.
- For example, in the U.S., manufacturers must comply with current Good Manufacturing Practices (cGMP) as mandated by the FDA under the Dietary Supplement Health and Education Act (DSHEA). Similarly, in Europe, regulations focus on safety, rigorous testing, and detailed documentation to ensure the quality of ingredients in nutraceuticals and dietary supplements.
As the complexity of regulatory requirements grows, companies are increasingly outsourcing production to specialized contract manufacturers who are well-versed in navigating these challenges, further driving market expansion.
Rising Consumer Demand for Health and Wellness Products
The growing consumer demand for health and wellness products is another major driver of the nutraceutical contract manufacturing services market. With a surge in awareness around preventive healthcare, more consumers are seeking natural and plant-based nutraceuticals, which are perceived to be safer and free from synthetic additives and preservatives. This shift toward natural, wellness-focused products is being fueled by a desire to improve overall health and maintain a balanced lifestyle.
- According to a report by eMarketer Inc. in December 2024, consumer spending on nutrition-based products in the U.S. increased by 38%. This shift in consumer preferences is significantly boosting the demand for nutraceutical products, especially those that are clean-label, plant-based, and organic.
As a result, companies in the nutraceutical industry are increasingly relying on contract manufacturers to meet the growing demand for high-quality products, making outsourcing a key factor in the sector’s growth.
Restraining Factor
Intellectual Property (ip) and Proprietary Formulation Risks
The outsourcing of production processes, especially to overseas facilities in regions with weaker patent enforcement or less stringent trade secret protections, raises concerns about the potential replication of formulas and unauthorized use of proprietary blends. These risks can significantly affect a company's ability to maintain a competitive edge and protect its innovations.
Moreover, outsourcing manufacturing often means relinquishing some degree of control over the production process, which can complicate efforts to maintain stringent quality assurance standards. Such loss of control may lead to inconsistencies in product quality or safety, undermining consumer trust and damaging a brand's reputation.
As a result, while third-party contract manufacturing can offer numerous benefits, companies must carefully manage these risks through robust IP safeguards, including strict non-disclosure agreements (NDAs) and carefully vetted partnerships. Without these protections in place, the risk of formula replication or the introduction of competing products could have long-lasting negative effects on both market position and profitability.
Market Opportunity
Increased Demand for Sustainability and Clean-Label Products
With heightened awareness of environmental and health concerns, consumers are increasingly seeking transparency in the products they purchase. Clean-label formulations, which emphasize natural, organic, and ethically sourced ingredients, have become a key expectation for today’s nutraceutical buyers. Moreover, consumers are especially focused on avoiding products with synthetic additives, preservatives, or genetically modified organisms, further driving the demand for natural, organic solutions.
- For example, the surge in popularity of organic ingredients is a notable trend within the nutraceutical sector. In fact, according to the Organic Trade Association, U.S. organic food sales reached $62 billion in 2020, reflecting a robust market demand for organic products. This trend is expected to continue, with the global organic food market projected to grow at a CAGR of 14% through 2027.
This shift in consumer preferences offers a prime opportunity for contract manufacturers specializing in organic sourcing and ensuring certified organic supply chains.
Regional Insights
North America holds a dominant position in the global nutraceutical contract manufacturing services market, driven by its well-established regulatory framework, advanced manufacturing infrastructure, and strong consumer demand for dietary supplements. The region benefits from a high concentration of GMP-certified contract manufacturers, state-of-the-art formulation technologies, and significant investments in personalized nutrition and clean-label products.
Moreover, the growing popularity of plant-based supplements, innovation in delivery formats such as gummies and liquid capsules, and the presence of major nutraceutical brands outsourcing production contribute to North America's market leadership. This combination of regulatory support, technological advancements, and a receptive consumer base positions North America as a key player in the nutraceutical manufacturing space.
The U.S. is the primary market for nutraceutical contract manufacturing services, driven by stringent FDA regulations, a well-developed nutraceutical industry, and high demand for dietary supplements. For instance, in March 2024, according to the Penn State Health News, approximately 59 million Americans use some type of vitamins or supplements regularly, spending an average of $510 annually. Moreover, Amazon’s private-label supplement sales grew by 40% in 2023, increasing outsourcing demand for contract manufacturers in the U.S.
Asia Pacific Nutraceutical Contract Manufacturing Services Market Trends
Asia-Pacific is emerging as the fastest-growing market for nutraceutical contract manufacturing services, with the highest projected CAGR. This growth is fueled by rising health awareness, increasing disposable incomes, and an expanding demand for herbal and traditional supplements. Key countries like China, India, and Japan are becoming prominent hubs for nutraceutical production, benefiting from cost-effective manufacturing, abundant raw materials, and government initiatives that support the industry.
China’s nutraceutical contract manufacturing services is a major market, with rising consumption of dietary supplements like vitamins, minerals, and functional foods. The country’s massive population and growing health awareness have led to a surge in demand for personalized and natural nutraceuticals. Moreover, e-commerce platforms have expanded product accessibility, while innovation in the industry creates new opportunities for contract manufacturers to offer advanced solutions tailored to Chinese consumers.
Japan is a leader in functional foods and precision nutrition, with a strong demand for fermented supplements, probiotics, and beauty-from-within products, such as collagen-based supplements. Companies like Kirin and Morinaga are driving innovation in probiotic formulations. Japan’s FOSHU (Foods for Specified Health Uses) approval system, regulating over 1,000 certified health foods, increases the need for contract manufacturers specializing in scientifically-backed, government-approved nutraceutical formulations.
India’s nutraceutical contract manufacturing market is rapidly growing, driven by cost-effective manufacturing and abundant raw materials. The rising adoption of Ayurveda-based supplements has fueled global expansion for companies like OmniActive Health Technologies. Government initiatives like the Production-Linked Incentive (PLI) scheme are further boosting investments in GMP-certified facilities, enhancing India’s position as a growing hub for the production of herbal and organic nutraceutical products.
Europe Nutraceutical Contract Manufacturing Services Market Trends
Germany, a major European market, has seen a growing demand for nutraceuticals, particularly products supporting immunity, cognitive performance, and overall well-being. Strong regulations, including compliance with GMP standards, make Germany an ideal location for contract manufacturers. The shift toward plant-based and organic supplements, along with a focus on clean-label products, is driving companies to collaborate with contract manufacturers to meet stringent EU regulations for high-quality production.
Formulation Insights
The tablets segment dominates the nutraceutical contract manufacturing services market, accounting for the highest market revenue. Their popularity is driven by factors like ease of manufacturing, longer shelf life, and versatility in design. Tablets can be customized into various sizes, shapes, and coatings, allowing manufacturers to cater to specific consumer preferences. Moreover, their cost-effectiveness and ability to retain potency over extended periods make them a preferred choice. The convenience of packaging and widespread acceptance further solidifies tablets as the leading segment.
Product Insights
The dietary supplements segment holds the largest market share in the nutraceutical contract manufacturing services market. This dominance is fueled by the growing consumer awareness surrounding health and wellness. The increasing preference for vitamins, minerals, probiotics, herbal supplements, and other functional ingredients has contributed to this trend. As individuals seek natural alternatives for overall health, immunity, and mental well-being, dietary supplements are becoming an essential part of daily routines. Consequently, the demand for high-quality, innovative dietary supplement formulations is driving growth.
Company Market Share
Key players in the nutraceutical contract manufacturing services market are increasingly focusing on adopting key business strategies to strengthen their market presence. These strategies include strategic collaborations with industry leaders to expand their service offerings and enhance technological capabilities.
Nutralab: An Emerging Player in the Market
NutraLab is a leading manufacturer specializing in vitamins and offering both OEM and private-label services for nutraceuticals and dietary supplements. With state-of-the-art production facilities certified by Health Canada and holding a GMP site license, NutraLab upholds the highest standards of quality assurance.
A subsidiary of Honson Pharmatech Group, the company operates across three strategic locations in Markham and Toronto, Ontario, with its flagship Toronto facility being recently upgraded to accommodate its expanding manufacturing capabilities.
Recent Developments at Nutralab Include:
- In December 2024, Nutralab was recognized with two prestigious awards at the 2024 Global Corporate Excellence Awards by Business Worldwide Magazine. Additionally, Dr. Peter Ou, the company's leader, was honored with the "Outstanding Leadership in Nutraceutical Innovation" award for their dedication to the industry.
List of Key and Emerging Players in Nutraceutical Contract Manufacturing Services Market
- Ashland
- Glanbia PLC
- Herbalife International of America, Inc.
- Biotrex Nutraceuticals
- Martínez Nieto
- Menadiona
- NUTRIVO
- American Health Foundations, Inc.
- Gemini Pharmaceuticals
- Biovencer Healthcare Pvt Ltd
- Rain Nutrience
- NutraLab
- Biofarma Group

to learn more about this report Download Market Share
Recent Developments
- In September 2024, Ashland Inc. finished the sale of its nutraceuticals business to an affiliate of Turnspire Capital Partners LLC, known as Turnspire, effective August 30, 2024. The transaction includes custom formulation and contract manufacturing capabilities in the nutrition market from the US production facilities in New Jersey and Utah and Tamaulipas, Mexico. Financial terms of the deal were not disclosed.
Analyst Opinion
As per our analyst, the global nutraceutical contract manufacturing services market is witnessing substantial growth, propelled by the escalating demand for personalized nutrition, clean-label, and functional supplements. Companies are increasingly outsourcing production to GMP-certified manufacturers to ensure regulatory compliance, cost efficiency, and access to innovative delivery technologies, such as liposomal encapsulation and probiotics.
With a strong emphasis on sustainable sourcing, clinical validation, and digitized supply chains, manufacturers are prioritizing quality and transparency, further fueling market expansion. Despite the opportunities, the market faces several challenges, including intellectual property risks, particularly when collaborating with overseas facilities, and the complexities of navigating global regulations.
Moreover, maintaining consistent product quality and meeting rising consumer expectations for clean-label and plant-based nutraceuticals can be demanding. Nonetheless, industry players are positioning themselves to thrive in a highly competitive and evolving market by addressing these challenges effectively through strategic collaborations, innovation, and compliance.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 182.47 Billion |
| Market Size in 2025 | USD 198.34 Billion |
| Market Size in 2033 | USD 386.6 Billion |
| CAGR | 8.7% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Formulations, By Product |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Nutraceutical Contract Manufacturing Services Market Segments
By Formulations
- Tablets
- Capsules
- Liquid
- Gummies
- Energy Bars
- Other Suitable Forms
By Product
-
Dietary Supplements
- Proteins & Amino Acids Supplement
- Weight Management and Meal Replacer Supplement
- Multivitamin, Multi-Mineral, and Antioxidant Supplement
- Other Supplements
- Functional Food and Beverages
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































