Nutraceutical Excipients Market Size, Share & Trends Analysis Report By Function (Binders and fillers, Disintegrants, Coatings and film formers, Lubricants and glidants, Sweeteners and flavour systems, Solubilisers and emulsifiers, Preservatives and antioxidants, Controlled-release polymers, Colorants, Other carriers), By Dosage Form (Tablets, Capsules, Gummies and chewables, Powders and sachets, RTD liquids and shots, Softgels and liquid-fill), By Source (Synthetic, Plant-based, Mineral, Animal-derived), By End User (Nutraceutical brand owners, Contract manufacturers/CDMOs, Private label/retail brands, Clinical nutrition companies) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Nutraceutical Excipients Market Overview
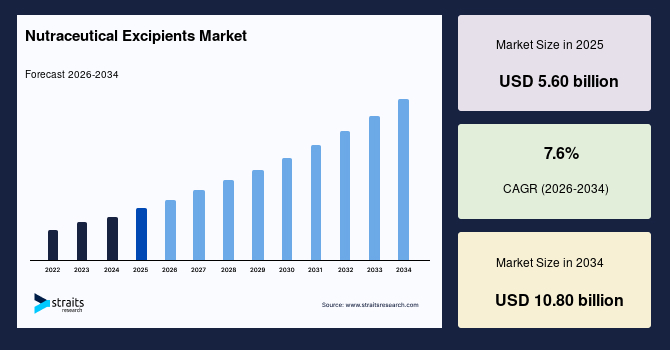
The global nutraceutical excipients market size is valued at USD 5.60 billion in 2025 and is estimated to reach USD 10.80 billion by 2034, growing at a CAGR of 7.60% during the forecast period. The market’s growth is supported by the reformulation of clean-label and low-sugar products, stricter labelling and compliant formats, and coating innovations that protect sensitive actives such as probiotics, enzymes, and botanicals.
Key Market Trends & Insights
- North America dominated the market with a revenue share of 37.4% in 2025.
- Asia Pacific is anticipated to grow at the fastest CAGR of 11.5% during the forecast period.
- Based on function, the binders and fillers segment held the highest market share of 27% in 2025.
- By Dosage Form, the gummies segment is estimated to register the fastest CAGR growth of 10.5%.
- Based on source, the Synthetic excipientscategory dominated the market in 2025 with a revenue share of 36%.
- Based on end user, the Contract Manufacturers or CDMOssegment is projected to register the fastest CAGR of 9.8% during the forecast period.
- U.S. dominates the market, valued at USD 1,173.61 million in 2024 and reaching USD 1,257.75 million in 2025.
Table: U.S. Nutraceutical Excipients Market Size (USD Million)
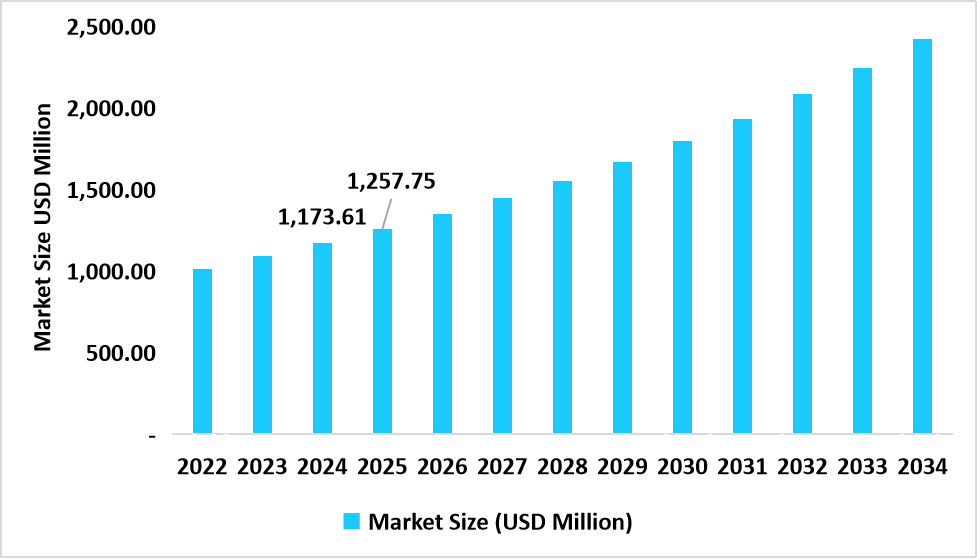
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 5.60 billion
- 2034 Projected Market Size: USD 10.80 billion
- CAGR (2026-2034): 7.60%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The nutraceutical excipients market covers binders, fillers, disintegrants, coatings, capsule materials, sweeteners, and texturizers formulated for dietary supplements and functional nutrition. The market demand is reinforced by policy pressure to limit sugars and improve transparency, which prompts brands to shift toward sugar-reduced gummies, shots, and tablets that utilise newer film formers, plant-based capsules, and cleaner carriers. As brands expand convenient formats and healthy-ageing use cases, excipients that enhance palatability, bioavailability, and protection become central to product performance and regulatory confidence.
Latest Market Trends
Clean-label, low-sugar reformulation elevates excipient performance
The nutraceutical industry is shifting toward cleaner labels and lower sugar content, driven by stricter regulations, such as the FDA's revised definition of "healthy," as well as global health trends. This pushes brands to cut traditional sugars and simplify their ingredient lists. As a result, excipients are challenged to offer a good taste, stability, and mouthfeel without added sugar, thereby increasing the demand for plant-based ingredients such as cellulose, natural colours, and advanced low- or no-sugar sweetener systems. Special coatings are also crucial for masking the strong flavours of botanicals or amino acids in sugar-free products. Suppliers offering clean-spec ingredients that also work smoothly on manufacturing lines are experiencing strong demand.
Format shift to gummies, shots, and vegan capsules
There is a shift in format towards Gummies and Ready-to-Drink (RTD) shots, as consumers are opting for more convenient and enjoyable supplement formats. These formats require specialised ingredients, such as alternative gelling agents like pectin instead of gelatin, and advanced flavour systems to deliver nutrients effectively. Simultaneously, there is a clear trend toward vegan capsules (HPMC) and specially designed liquid-filled formats for clean labels. This shift boosts demand for high-quality cellulose, modified starches, and functional coatings that can meet both consumer preference for clean labels and complex technical needs.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 5.60 billion |
| Estimated 2026 Value | USD 6.01 billion |
| Projected 2034 Value | USD 10.80 billion |
| CAGR (2026-2034) | 7.6% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Roquette (incl. Qualicaps), Evonik Health Care, Ashland, Colorcon, BASF Nutrition & Health / LDC Ingredients (post-transaction) |
to learn more about this report Download Free Sample Report
Market Drivers
Strategic investments and portfolio upgrades accelerate supply and innovation
Nutraceutical excipient suppliers are expanding their capacity and range to meet the evolving needs of the rapidly growing supplement market.
- For example, in April 2025, Ashland expanded its coating production facility in Brazil, helping brands in the Americas access film-coating systems more quickly and at lower logistics costs.
- Similarly, in September 2025, BASF deepened its partnership network to secure more plant-based excipient inputs, supporting the growing demand for clean-label and vegan formulations.
These upgrades support faster product turnaround and improve reliability for brands scaling gummies, RTD beverages, and vegan capsules. Coupled with flavour and coating collaborations, these moves reduce production hurdles and enable more stable, high-performing formats that meet consumer expectations for taste, sustainability, and quality.
Regulatory clarity and quality signals favour established excipient systems
The FDA’s ongoing updates on the New Dietary Ingredient (NDI) process are increasing the focus on quality, identity testing, and safety for all supplement components. This regulatory clarity gives an advantage to excipient suppliers who provide detailed data, adhere to pharmaceutical standards, and have proven stability data for different product types (like gummies or liquids). Clearer rules reduce risk for formulators and expedite the launch of new products, particularly in low-sugar and novel delivery formats. Brands are increasingly opting for multifunctional excipients to enhance manufacturing efficiency and ensure compliance.
Market Restraint
High cost of premium excipient grades limits widespread adoption
One of the key restraints in the nutraceutical excipients market is the higher cost of advanced, clean-label, or speciality-grade excipients. These include plant-based capsules, targeted-release coatings, and low-nitrite cellulose derivatives that meet stricter safety or labelling standards. While large nutraceutical brands can justify these costs for premium or clinical products, smaller companies with tighter budgets struggle to absorb the added expense. The result is a slower adoption of safer and more advanced excipient options in mass-market supplements. This cost barrier can restrict innovation and prevent the broader availability of high-quality, compliant formulations in emerging markets.
Market Opportunity
Next-gen protective capsules and targeted-release coatings
Advanced next-gen capsules and targeted release coatings offer new opportunities for delivering probiotics, enzymes, and herbal actives. Targeted-release capsules enable brands to precisely deliver active ingredients to the intestine, thereby enhancing the effectiveness of probiotics and enzymes.
- For instance, in October 2025, Evonik announced the launch of its EUDRACAP® colon functional capsules under Good Manufacturing Practice (GMP) quality for clinical and commercial use. This allows for the targeted release of sensitive active ingredients in the ileo-colonic region of the gut
These advanced systems are crucial for high-growth areas, including gut health, women's health, and healthy ageing. By using these shells along with low-nitrite coatings, brands can make specific, science-backed claims about release profiles, which helps them stand out in crowded supplement aisles.
Regional Analysis
North America dominated the market in 2025, accounting for 37.40% market share due to mature supplement manufacturing, strong retail and DTC penetration, and clearer compliance pathways that reduce launch risk. The U.S. FDA’s guidance on supplement testing and documentation maintains high quality, favouring proven excipient suppliers. This boosts demand for advanced ingredients, such as plant-based coatings, taste-masking systems, and alternative sweeteners, used in low-sugar gummies, tablets, and shots, making the region a hub for both volume and quality innovation.
- The U.S. is the central innovation hub, featuring large contract manufacturers and frequent regulatory updates. FDA resources reinforce standards, leading brands to use certified binders, coatings, and capsules. As products cut sugar to meet "healthy" labelling trends, the demand for plant-based film formers and taste-masking systems increases, reinforcing the U.S. as the primary source of volume and innovation.
Asia Pacific's Market Trends
Asia Pacific is emerging as the fastest-growing region with a CAGR of 11.5% from 2026-2034, driven by urban demand for convenient, low and no-sugar functional products and by regulatory nudges that favour transparent labelling. Regional co-manufacturing capacity for gummies and capsules is expanding, while cross-border e-commerce spreads clean-label and vegan capsule preferences. These dynamics widen adoption of HPMC capsules, pectin or modified-starch gummy systems, and rapid film coats designed for humidity stability across tropical markets, fueling APAC’s outsized growth trajectory from a smaller base.
- Singapore leads innovation and acts as a regulatory model. Nutri-Grade rules incentivise low-sugar products, prompting brands to refine sweeteners and coatings while maintaining product palatability. Due to Singapore’s major role in retail and distribution, compliant, well-formulated products like RTD shots and chewables can quickly scale across the region, boosting demand for high-quality, plant-based excipients.
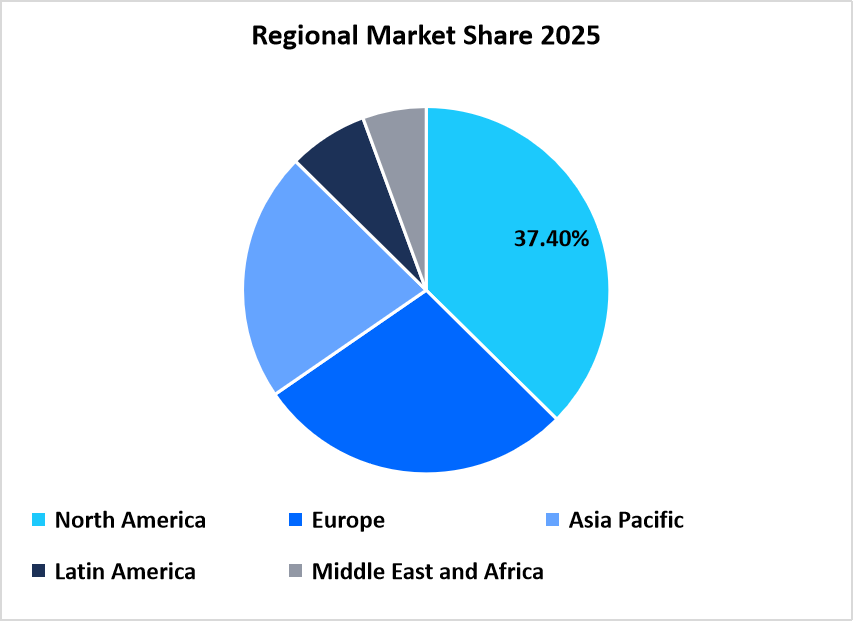
Source: Straits Research
European Market Trends
Europe is a high-value market defined by strict quality standards and a focus on advanced delivery systems. Brands often utilise specialised capsules and coatings to safeguard sensitive ingredients, such as probiotics, while meeting clean-label requirements. A key highlight is the availability of new, advanced capsules that can target the colon for better delivery of certain actives, showing Europe's leadership in engineering functional dosage forms. The strong push for evidence-based and low-sugar products ensures consistent demand for high-performing taste-masking agents and quality excipients, such as HPMC and ethylcellulose.
- Germany's advanced ecosystem of excipient suppliers and strict quality expectations supports the use of high-specification materials and complex capsules. The availability of GMP-produced functional capsules locally allows German contract manufacturers to quickly adopt pharmaceutical-grade delivery systems (like colon-targeted shells) for consumer health products, accelerating market launches for differentiated, clean-label European brands.
Latin American Market Trends
Latin America is driven by new supplement regulations and policies aimed at reducing sugar and promoting clearer labelling. Brazil’s ANVISA updated its rules in 2025, clarifying requirements for components and usage limits, which helps formulators comply faster. Mexico’s ongoing front-of-pack labelling rules continue to push for reformulation. As brands standardise labels and cut sugar, they increasingly use natural colours, plant-based film formers, and taste-masking agents, with modern retail channels supporting the scale-up of these new formulations.
- Brazil acts as the region’s regulatory and manufacturing anchor. The 2025 ANVISA updates provide clear compliance pathways for excipients and claims. With a large pharmacy network and growing demand for functional beverages, local manufacturers are adopting modern formats, such as pectin-based gummy systems and HPMC capsules, for their sugar-reduced products, which supports quicker commercialisation and potential regional export growth.
Middle East and Africa (MEA) Market Trends
The Middle East and Africa market demand is being driven by government policies that focus on sugar reduction and clearer nutrition facts. The UAE’s tiered sugar-based excise tax strongly encourages low- or no-sugar beverages and shots, which rely heavily on advanced sweetener systems and emulsifiers. As modern trade expands and the hot climate requires better stability, the need for high-quality coatings and moisture-controlled capsules increases. The localisation of low-sugar functional products increases demand for plant-based excipients and taste-maskers, starting from a relatively smaller base.
- The UAE’s new tiered tax directly rewards sugar reduction in beverages, pushing brands to innovate with compliant, low-sugar energy and focus drinks. This policy is expected to increase shelf space for functional shots and supplements. The local environment increases the demand for premium sweeteners, high-performance flavour systems, and plant-based film coatings that are specifically designed to withstand the challenging Gulf climate.
Function Insights
Binders and fillers dominated the market with a revenue share of 27% in 2025, due to their universal role in tablets and capsules, the most common supplement formats. Materials like microcrystalline cellulose (MCC) and dicalcium phosphate (DCP) provide consistent flow, compressibility, and content uniformity. Even as brands experiment with new delivery formats, binders and fillers remain essential for dose stability and tablet integrity, ensuring they continue to anchor excipient spend globally.
Controlled-release polymers segment is the fastest growing, exhibiting a CAGR of 10.2%, as brands seek to differentiate through targeted or sustained delivery. HPMC and ethylcellulose coatings help improve the stability of sensitive actives, such as probiotics and enzymes, reduce gastric discomfort, and enable colon- or intestine-specific release. These materials also support clean-label demands and low-nitrite requirements by offering plant-based or safer grades.
Dosage Form Insights
Tablets hold the largest market share of 34% due to their low manufacturing costs, scalability, and robust shelf life. They work well in varied climates, support scoring for dose flexibility, and require fewer excipients compared to gummies or RTDs. With mature supply chains for compression-grade excipients and rapid coating systems, tablets will continue to dominate core categories such as multivitamins, minerals, and condition-specific blends.
Gummies are the fastest-growing segment, with a CAGR of 10.5%, due to their taste, convenience, and appeal among younger and female consumers. Advances in pectin-based and vegan formulations support clean-label and allergen-free claims, enhancing the credibility of these formulations. Brands use bold flavours, creative shapes, and beauty- or immunity-focused actives to justify premium pricing. As retail space for chewables expands and consumers increasingly choose “fun” supplement formats, gummies are gaining momentum.
By Dosage Form Market Share (%), 2025
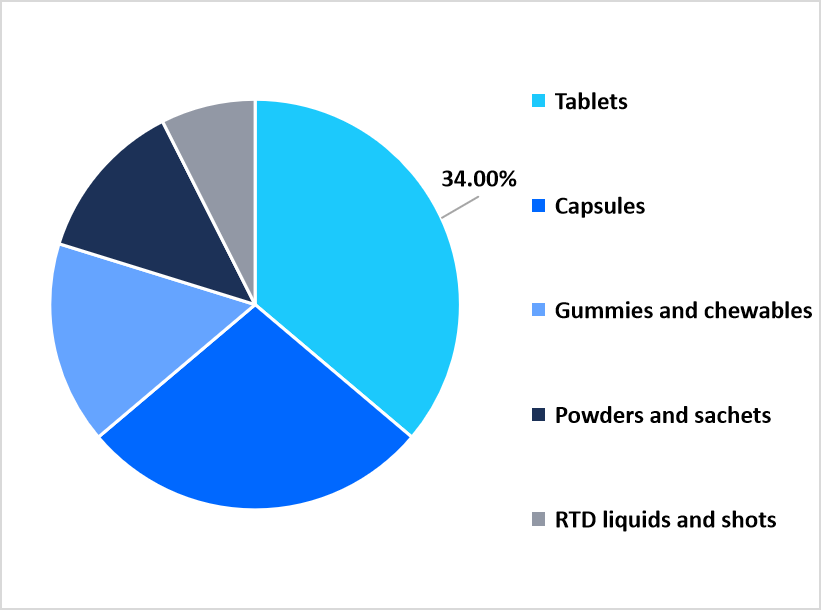
Source: Straits Research
Source Insights
Synthetic excipients lead the market, holding a revenue share of 36%. They are preferred due to their consistency, purity, and proven track record of performance in large-scale manufacturing. They ensure reliable compaction, flow, and disintegration across batches, minimising risks during global launches. With strong pharmacopeial backing and worldwide availability, synthetics enable brands to optimise production efficiency and dual-source materials for reduced supply risk. Even as plant-based demand grows, synthetics remain the backbone for high-speed tablet, capsule, and coating applications.
Plant-based excipients are the fastest-growing segment, with a CAGR of 9.1%, driven by rising demand for vegan, clean-label, and allergen-free products. HPMC capsules commonly replace gelatin shells, while starches and pectins enable the production of organic and sugar-reduced gummies. These ingredients align with retailer and ESG mandates, offering traceable, palm-free, and low-nitrite options. Advances in plant-derived delayed-release coatings are also removing technical barriers, making them suitable for complex formulations, such as probiotics and botanicals.
End User Insights
Nutraceutical Brand Owners dominate the market with a revenue share of 46% in 2025. They control product portfolios, reformulation timelines, and market launches. They rely on excipients to meet evolving clean-label, low-sugar, and performance goals while maintaining manufacturing efficiency. Their scale allows them to co-develop proprietary compression blends or capsule shells directly with suppliers.
Contract Manufacturers or CDMOs are growing at the fastest rate, with a CAGR of 9.8% in 2025, as brands outsource complex, multi-format production. They offer formulation expertise, regulatory support, and turnkey solutions for gummies, delayed-release shells, and microencapsulated actives. Their ability to scale novel delivery formats and meet retailer quality expectations makes them an attractive choice for both established and emerging supplement brands.
Competitive Landscape
The global nutraceutical excipients market is moderately fragmented, featuring a mix of multinational excipient manufacturers, capsule and coating specialists, flavour and texture system experts, and regional CDMOs. A long tail of regional and niche players continues to compete in areas like taste-masking, targeted release, or vegan capsules. Key differentiators include GMP-grade quality, clean-label options (such as low-nitrite cellulose), and scalability in fast-growing formats like gummies and RTDs.
Roquette Pharma Solutions: An Emerging Player
Roquette Pharma Solutions is emerging as a key integrated partner following the acquisition of IFF Pharma Solutions and Qualicaps. This move gives Roquette end-to-end control over plant-based binders, coatings, sweeteners, and HPMC capsule shells, aligned with strong demand for clean-label and vegan formats. The wider footprint improves customer collaboration, supporting brands from prototype to commercial launch in tablets, gummies, and modified-release capsules.
Latest News
- In August 2025, Roquette launched METHOCEL™ Low-Nitrite HPMC (≤200 ppb), a safer-by-design excipient that helps supplement brands comply with rising global nitrosamine controls. The product immediately garnered industry attention for its clean-label use in gummy, tablet, and capsule formulations.
List of Key and Emerging Players in Nutraceutical Excipients Market
- Roquette (incl. Qualicaps)
- Evonik Health Care
- Ashland
- Colorcon
- BASF Nutrition & Health / LDC Ingredients (post-transaction)
- DFE Pharma
- JRS Pharma
- MEGGLE
- Shin-Etsu Chemical
- Gattefossé
- Lubrizol Life Science – Health
- Ingredion
- Kerry Group
- Tate & Lyle
- DSM-Firmenich
- Lonza Capsules & Health
- ACG
- Qualicaps
- CapsCanada
- IMCD
- SPI Pharma
- DuPont/IFF
Strategic Initiatives
- April 2025 - Ashland completed a USD 10 million expansion at Cabreúva, adding Aquarius tablet-coating and microbial-protection capability to support the Americas' supply and faster launches of sugar-reduced tablets.
- May 2025 - Roquette completes acquisition of IFF Pharma Solutions, creating a larger excipient platform spanning cellulose derivatives, binders, and capsules, strengthening support for nutraceutical delivery and clean-label needs.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 5.60 billion |
| Market Size in 2026 | USD 6.01 billion |
| Market Size in 2034 | USD 10.80 billion |
| CAGR | 7.6% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Function, By Dosage Form, By Source, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Nutraceutical Excipients Market Segments
By Function
- Binders and fillers
- Disintegrants
- Coatings and film formers
- Lubricants and glidants
- Sweeteners and flavour systems
- Solubilisers and emulsifiers
- Preservatives and antioxidants
- Controlled-release polymers
- Colorants
- Other carriers
By Dosage Form
- Tablets
- Capsules
- Gummies and chewables
- Powders and sachets
- RTD liquids and shots
- Softgels and liquid-fill
By Source
- Synthetic
- Plant-based
- Mineral
- Animal-derived
By End User
- Nutraceutical brand owners
- Contract manufacturers/CDMOs
- Private label/retail brands
- Clinical nutrition companies
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Anantika Sharma
Research Practice Lead
Anantika Sharma is a research practice lead with 7+ years of experience in the food & beverage and consumer products sectors. She specializes in analyzing market trends, consumer behavior, and product innovation strategies. Anantika's leadership in research ensures actionable insights that enable brands to thrive in competitive markets. Her expertise bridges data analytics with strategic foresight, empowering stakeholders to make informed, growth-oriented decisions.










































