Oligonucleotide CDMO Market Size, Share & Trends Analysis Report By Service Type (Contract Manufacturing, Contract Development), By Type (Antisense Oligonucleotides, Small Interfering RNA, Other Oligonucleotides), By Application (Therapeutic, Research, Diagnostic), By End Use (Pharmaceutical and Biopharmaceutical Companies, Diagnostic Companies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Oligonucleotide CDMO Market Overview
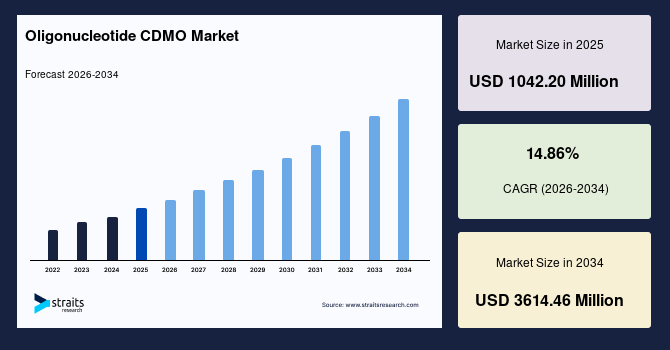
The global oligonucleotide CDMO market size is valued at USD 1042.20 million in 2025 and is estimated to reach USD 3614.46 million by 2034, growing at a CAGR of 14.86% during the forecast period. The consistent growth of the market is augmented by the increasing outsourcing of complex oligonucleotide synthesis and manufacturing activities as pharmaceutical and biotechnology companies advance RNA-based therapeutic pipelines and seek specialized external capabilities to support development scale up and commercial production.
Key Market Trends & Insights
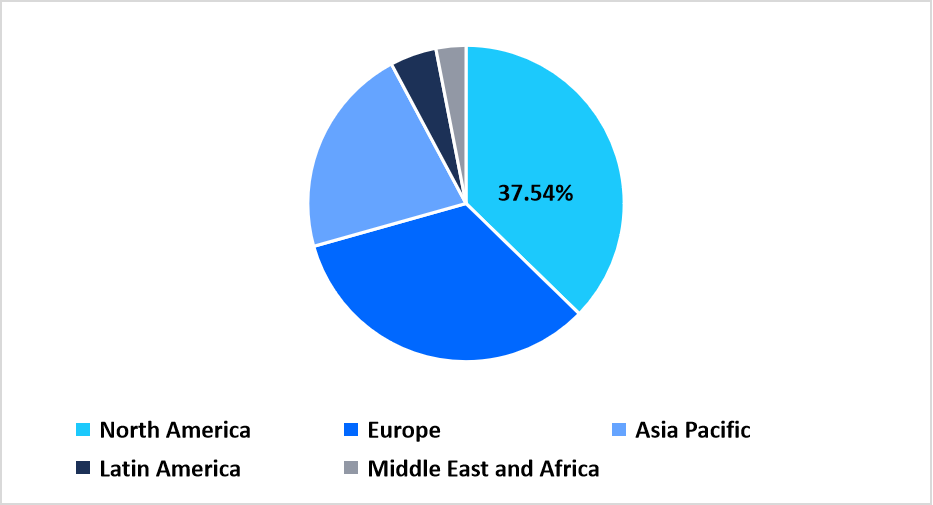
- North America held a dominant share of the global market, accounting for 37.54% in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 16.86% during the forecast period.
- Based on Service Type, contract development is anticipated to register the fastest CAGR of 15.23% during the forecast period.
- Based on Type, antisense oligonucleotides dominated the market with a revenue share of 36.78%.
- Based on the Application, the therapeutic segment dominated the market with a revenue share of 39.85% in 2025.
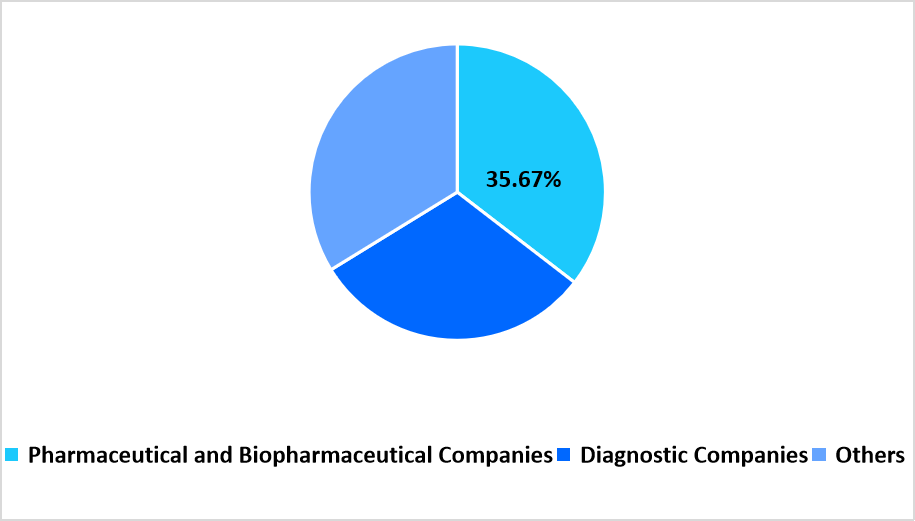
- Based on End Use, pharmaceutical and biopharmaceutical companies dominated the market with a revenue share of 35.67%.
- The U.S. dominates the market, valued at USD 308.91 million in 2024 and reaching USD 353.49 million in 2025.
Table: U.S. Oligonucleotide CDMO Market Size (USD Million)
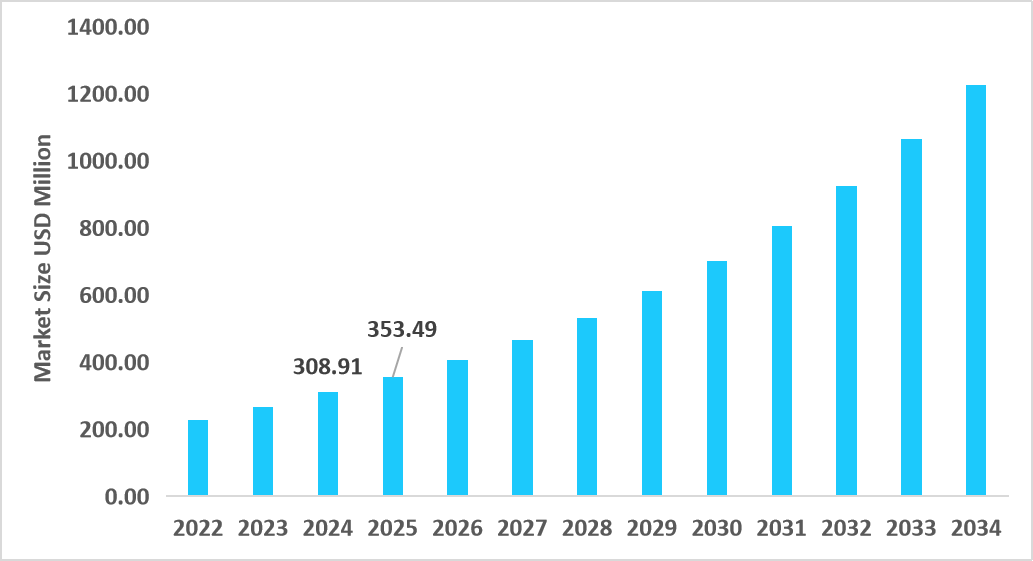
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1042.20 million
- 2034 Projected Market Size: USD 3614.46 million
- CAGR (2026-2034): 14.86%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The oligonucleotide CDMO market comprises specialized contract organizations that provide outsourced development and manufacturing services for synthetic oligonucleotides used across pharmaceutical, biotechnology, and diagnostic applications. These CDMOs support clients throughout the product lifecycle, covering early stage process development, analytical characterization, scale up, and GMP compliant commercial production of oligonucleotide drug substances and intermediates. The market is segmented by service type into contract manufacturing and contract development, reflecting demand across both production focused and development focused engagements. By type, the market includes antisense oligonucleotides, small interfering RNA, and other oligonucleotides designed for diverse molecular mechanisms. By application, oligonucleotide CDMO services address therapeutic, research, and diagnostic uses, driven by expanding adoption of nucleic acid based technologies. By end use, the market serves pharmaceutical and biopharmaceutical companies, diagnostic companies, and other research driven organizations that outsource complex synthesis and regulatory aligned manufacturing activities to specialized partners.
Latest Market Trends
Shift from Generalized Urinary Wellness Formulations to Condition Specific Supplementation
A defining trend in the urology supplements market is the shift from generalized urinary wellness formulations to condition specific supplementation targeting prostate health, bladder control, kidney function, and male reproductive wellness. Manufacturers increasingly design formulations tailored to distinct urological conditions using targeted ingredient combinations and dosage profiles. This shift reflects growing consumer awareness of differentiated urological needs across age groups and clinical presentations, positioning specificity as a core factor influencing product selection and brand positioning.
Shift from Single Ingredient Products to Multi Component Botanical Blends
The notable trend is the shift from single ingredient products to multi component botanical blends that combine plant extracts, micronutrients, and bioactive compounds. Supplement developers increasingly focus on synergistic formulations that address multiple urological pathways within a single product. This transition supports broader functional positioning and aligns with consumer preference for comprehensive supplementation approaches rather than isolated nutrient intake.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1042.20 Million |
| Estimated 2026 Value | USD 1193.32 Million |
| Projected 2034 Value | USD 3614.46 Million |
| CAGR (2026-2034) | 14.86% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | PolyPeptide Group, STA Pharmaceutical Co. Ltd., Bachem, Creative Peptides, Aurigene Pharmaceutical Services Ltd. |
to learn more about this report Download Free Sample Report
Oligonucleotide CDMO Market Driver
Rising Self Directed Management of Chronic Urological Conditions
The urology supplements market is driven by increasing adoption of self directed management approaches for chronic urological conditions such as benign prostatic enlargement and urinary discomfort. Consumers increasingly seek non prescription supplementation as part of long term wellness routines, supporting steady demand for urology focused dietary products across retail and online distribution channels.
Market Restraint
Variability in Clinical Substantiation Across Supplement Formulations
A key restraint in the urology supplements market is variability in clinical substantiation across commercially available formulations. Differences in ingredient sourcing, concentration levels, and formulation standards create uncertainty among healthcare professionals and informed consumers, limiting broader recommendation within clinical advisory settings.
Market Opportunity
Development of Urology Supplements Aligned with Preventive Health Programs
A notable opportunity exists in the development of urology supplements aligned with preventive health programs and routine wellness screening initiatives. Increasing focus on early intervention for age related urological changes creates potential for supplements positioned around long term maintenance and preventive use, supporting market expansion through proactive health management strategies.
Regional Analysis
North America accounted for the largest revenue share of the oligonucleotide CDMO market in 2025 with 37.54% share, supported by advanced drug development pipelines focused on RNA based therapeutics. Strong integration between biotechnology sponsors and contract manufacturers enables accelerated transition from early development to commercial scale production. Concentration of clinical stage antisense and siRNA programs sustains consistent outsourcing demand.
The U.S. oligonucleotide CDMO market is driven by high sponsor reliance on external manufacturing for complex chemistries, including modified backbones and conjugated oligonucleotides. Expansion of dedicated GMP oligonucleotide facilities supports late stage clinical supply and commercial readiness across rare disease and neurology programs.
Asia Pacific Market Insights
Asia Pacific is projected to record the fastest growth rate of 16.86% during the forecast period due to rising investment in nucleic acid therapeutics manufacturing infrastructure. Regional CDMOs expand capacity to serve global pharmaceutical clients seeking diversified supply chains and cost optimized production models. Increasing regulatory alignment with international quality standards enhances regional credibility.
China oligonucleotide CDMO market growth is driven by rapid scaling of domestic synthesis capabilities combined with rising participation in global clinical development programs. Government supported life science industrial zones enable high throughput oligonucleotide production and analytical testing services.
Regional Market share (%) in 2025
Source: Straits Research
Europe Market Insights
Europe demonstrates steady expansion supported by strong academic to industry translation in RNA research and structured regulatory pathways for advanced therapeutics. Regional CDMOs focus on complex sequence synthesis, impurity control, and batch consistency to support clinical and commercial supply requirements.
Germany oligonucleotide CDMO market is driven by emphasis on precision manufacturing and quality by design frameworks. Long standing expertise in chemical synthesis and process validation supports partnerships with multinational pharmaceutical companies pursuing late phase oligonucleotide programs.
Latin America Market Insights
Latin America experiences gradual market development supported by increasing participation in early phase clinical manufacturing and analytical support services. Regional activity remains concentrated in specialized contract manufacturers serving international sponsors for pilot scale production.
Brazil oligonucleotide CDMO market is driven by expanding clinical research infrastructure and local production incentives for advanced therapeutics. Collaboration between public research institutions and private CDMOs supports incremental growth in oligonucleotide development services.
Middle East and Africa Market Insights
The Middle East and Africa market is at a developing stage, with growth supported by selective investments in pharmaceutical manufacturing diversification. Adoption remains focused on technology transfer and formulation support rather than large scale synthesis.
Saudi Arabia oligonucleotide CDMO market is driven by national biotechnology initiatives aimed at localizing advanced drug manufacturing capabilities. Strategic partnerships with global CDMOs support workforce training and gradual capability expansion.
Service Type Insights
The contract manufacturing segment dominated the oligonucleotide CDMO market in 2025, supported by rising outsourcing of large scale GMP production for clinical and commercial oligonucleotide pipelines. Sponsors increasingly rely on external manufacturing partners to manage complex synthesis workflows, purification processes, and regulatory compliance, driving sustained revenue contribution from this segment.
The contract development segment is anticipated to register the fastest growth during the forecast period, accounting for a growth share of 15.23%. Growth is driven by expanding early stage oligonucleotide pipelines that require process optimization, analytical method development, and scale up support prior to clinical advancement.
Type Insights
The antisense oligonucleotide segment dominated the market in 2025 with a revenue share of 36.78%. Dominance is attributed to the maturity of antisense platforms, higher number of approved therapies, and consistent demand for development and manufacturing services across neurological and rare disease programs.
The small interfering RNA segment is projected to witness the fastest growth of 15.98% during the forecast period. Expansion is supported by rising clinical activity in gene silencing therapies and increasing sponsor focus on lipid conjugation and delivery-optimized siRNA constructs.
Application Insights
The therapeutic segment dominated the oligonucleotide CDMO market in 2025, representing a revenue share of 39.85%. Strong clinical translation of oligonucleotide-based drugs and growing late-stage pipelines support sustained outsourcing demand for therapeutic manufacturing services.
The diagnostic segment is expected to grow at the fastest rate with a growth share of 15.78%. Growth is driven by expanding use of synthetic oligonucleotides in molecular diagnostics, assay controls, and companion diagnostic development.
End Use Insights
The pharmaceutical and biopharmaceutical companies segment dominated the market in 2025 with a revenue share of 35.67%. High outsourcing intensity, complex regulatory requirements, and diversified therapeutic pipelines support the dominance of this end-use category.
The diagnostic companies segment is anticipated to register the fastest growth, accounting for a growth share of 15.89%. Expansion is supported by rising demand for custom oligonucleotide synthesis and quality-controlled production for diagnostic applications.
By End Use Market Share (%), 2025
Source: Straits Research
Competitive Landscape
The global oligonucleotide CDMO market is moderately fragmented, with a combination of large scale pharmaceutical service providers and specialized nucleic acid manufacturers operating across development and commercial manufacturing stages. Market participants compete on synthesis scale, regulatory compliance, analytical depth, and ability to manage complex chemistries across multiple therapeutic modalities.
Lonza: An Emerging Market Player
Lonza holds a strong position in the oligonucleotide CDMO market through its integrated development and manufacturing services for antisense and RNA based therapeutics. The company focuses on end to end project support spanning process development, scale up, and GMP production. Its capabilities include handling of modified nucleotides, impurity profiling, and regulatory documentation aligned with global submission standards. Lonza emphasizes long term partnerships with biotechnology sponsors advancing late stage clinical programs. Continued investment in capacity expansion, automation, and quality systems supports its growing role in supplying oligonucleotide drug substances for commercial launch programs.
List of Key and Emerging Players in Oligonucleotide CDMO Market
- PolyPeptide Group
- STA Pharmaceutical Co. Ltd.
- Bachem
- Creative Peptides
- Aurigene Pharmaceutical Services Ltd.
- Merck KGaA
- EUROAPI
- Curia Global, Inc.
- CordenPharm
- Sylentis, S.A.
- Thermo Fisher Scientific Inc.
- Agilent Technologies, Inc.
- Lonza
- PolyPeptide Group
- Creative Biogene
- Others
Strategic Initiatives
- July 2024: CordenPharma announced to invest USD 1061.24 million, which aimed to expand its peptide platform, both at its Colorado, U.S. site and in Europe.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1042.20 Million |
| Market Size in 2026 | USD 1193.32 Million |
| Market Size in 2034 | USD 3614.46 Million |
| CAGR | 14.86% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Service Type, By Type, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Oligonucleotide CDMO Market Segments
By Service Type
- Contract Manufacturing
- Contract Development
By Type
- Antisense Oligonucleotides
- Small Interfering RNA
- Other Oligonucleotides
By Application
- Therapeutic
- Research
- Diagnostic
By End Use
- Pharmaceutical and Biopharmaceutical Companies
- Diagnostic Companies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































