Oncology Clinical Trials Market Size, Share & Trends Analysis Report By Phase (Phase I, Phase II, Phase III, Phase IV), By Type (Breast Cancer, Melanoma Cancer, Colorectal Cancer, Prostate Cancer, Lung Cancer, Others), By Study (Interventional studies, Observational studies, Expanded access studies) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Oncology Clinical Trials Market Overview
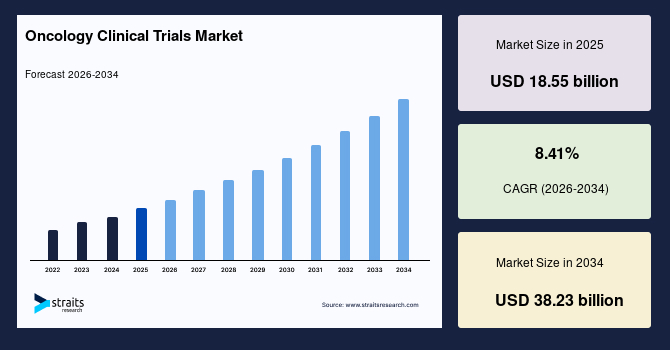
The global oncology clinical trials market size is estimated at USD 18.55 billion in 2025 and is projected to reach USD 38.23 billion by 2034, growing at a CAGR of 8.41% during the forecast period. Remarkable growth of the market is propelled by the advancements in the immune oncology sector and the rising focus on personalized medicine, which has created strong demand for trials assessing targeted treatments and combination therapies.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 47.92% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 9.52%, during the forecast period of 2026 to 2034.
- Based on Phase, the Phase I segment dominated the market in 2025 with a revenue share of 35.26%.
- Based on Type, the prostate cancer segment is expected to register the fastest CAGR growth of 9.12% during the forecast period of 2026 to 2034.
- Based on the Study, the interventional studies segment dominated the market in 2025, with a revenue share of 75.63%.
- The U.S. dominates the global market, valued at USD 7.40 billion in 2024 and reaching USD 7.99 billion in 2025.
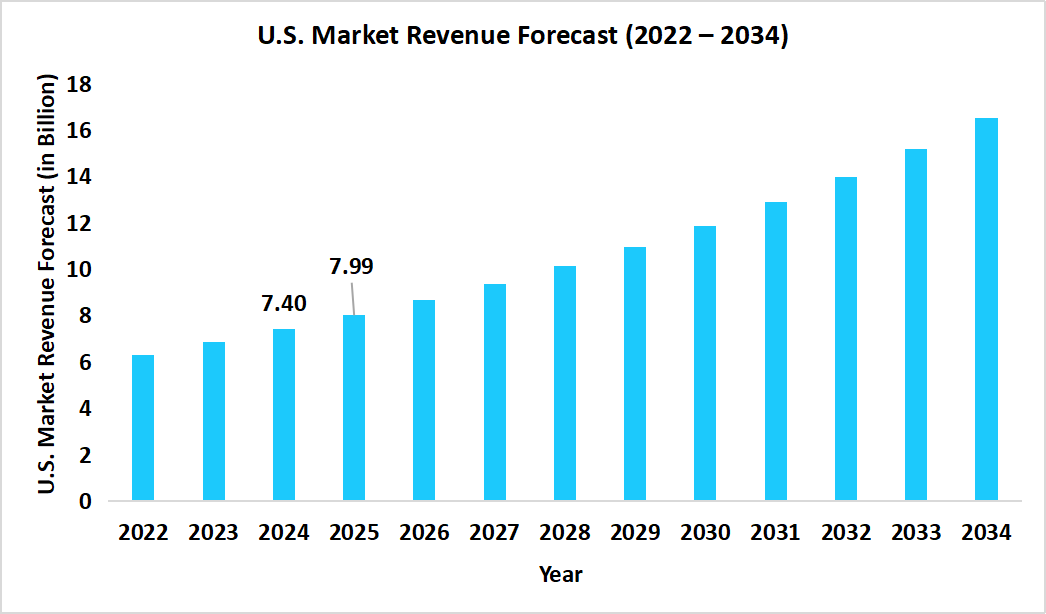
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 18.55 billion
- 2034 Projected Market Size: USD 38.23 billion
- CAGR (2025 to 2034): 8.41%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The global market encompasses studies focused on evaluating innovative cancer therapeutics and treatment approaches across multiple cancer types, including breast, melanoma, colorectal, prostate, lung, and other malignancies. These trials span across different phases, such as Phase I, Phase II, Phase III, and Phase IV, each playing a critical role from initial safety assessment to post-marketing evaluation. Based on study design, oncology clinical trials are classified into interventional, observational, and expanded access studies, aimed at assessing new therapies, monitoring outcomes, and providing investigational treatments. The market ecosystem comprises pharmaceutical and biotechnology companies, clinical research organizations, academic institutes, and regulatory agencies that collectively drive clinical development, streamline patient recruitment, and support the advancement of targeted, immune oncology, and combination therapies to improve cancer treatment outcomes.
Latest Market Trends
Cell & Gene Therapies Drive a Surge in Oncology Trial Activity
Oncology research moved strongly toward cell and gene therapies (CGT), with CAR T and TCR platforms expanding beyond a few blood cancers into new tumor types. Regulatory bodies have continued to approve novel cell-based therapies, while numerous early-phase studies have been initiated globally. The FDA’s Oncology Center of Excellence (OCE) and CBER reported approvals of new cell-based products, many tied to precision diagnostics, while ClinicalTrials.gov showed a high number of ongoing CAR T/TCR studies across global sites.
This trend encouraged more phase 1/2 pipelines and fostered growth in multicentre trial networks. As more companies invested in CGT programs, the momentum enhanced the oncology clinical trials market, expanding innovation and increasing patient access to next-generation therapies.
Radiopharmaceutical Therapies Expanded Oncology Trial Horizons
The oncology landscape shifted from a niche use of radiopharmaceuticals to a broader pipeline across many cancers. Once limited to prostate and neuroendocrine tumors, radioligand therapies (RLTs) are now tested in earlier treatment lines, in combinations, and in new indications. Novartis AG reported in its annual report that the global expansion of Pluvicto and its acquisition of Mariana Oncology resulted in a strong commitment to radioligand innovation.
This momentum led more sponsors to design dose-finding and multicenter studies, which widened trial scope and patient groups. As more pharma companies invested in radiopharmaceutical development, the trend boosted the growth of the global market, driving innovation and opening more treatment options.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 18.55 billion |
| Estimated 2026 Value | USD 20.04 billion |
| Projected 2034 Value | USD 38.23 billion |
| CAGR (2026-2034) | 8.41% |
| Dominant Region | Asia-Pacific |
| Fastest Growing Region | North America |
| Key Market Players | Hoffmann-La Roche Ltd, AstraZeneca, Merck & Co., Inc., Gilead Sciences, Inc , PARAXEL International Corporation |
to learn more about this report Download Free Sample Report
Market Driver
Robust Oncology Drugs Pipeline by Domestic Manufacturers
A key driver in the oncology clinical trials market is the strong pipeline of oncology drugs developed by domestic manufacturers, which fuels continuous clinical research and innovation in cancer therapies.
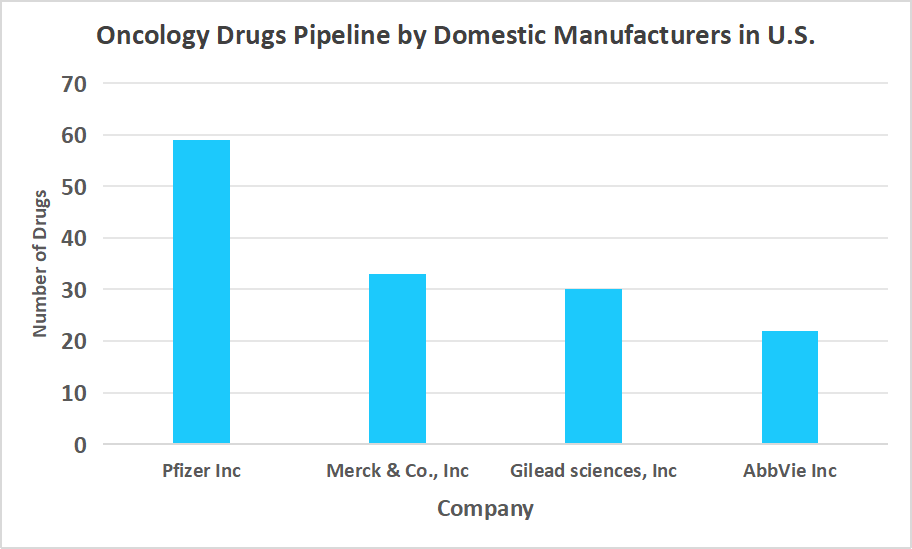
Source: Straits Research
Such a factor demonstrated a strong pipeline that supported market growth and expanded treatment options for patients.
Pharmaceutical Investments Fueling Oncology Market Expansion
Investments by pharmaceutical companies to expand oncology research and manufacturing facilities are propelling market growth, as detailed in the table below.
Table: Investment by Pharmaceutical Companies to Expand Cancer Research and Manufacturing Facilities in the U.S.
|
Date |
Company |
Investment (USD Million) |
Reason for Investment |
|
February 2024 |
AstraZeneca |
300 |
Critical cancer trials and future commercial supply |
|
July 2024 |
BeiGene |
800 |
Expand manufacturing capacity and clinical development capabilities for novel cancer treatments |
|
October 2024 |
Merck KGaA |
75 |
Expansion of antibody-drug conjugates manufacturing for novel cancer therapies |
Source: Straits Research
Such strategic investments, combined with the aforementioned growth factors, are driving the global market during the analysis timeframe.
Market Restraint
High Cost and Complexity of Conducting Oncology Clinical Trials
A challenge in the oncology clinical trials market is the escalating cost and complexity associated with conducting these studies. For example, in February 2024, the National Cancer Institute (NCI) highlighted that cancer trials were expensive to launch and operate, with numerous tests, lab work, and other protocol requirements extending study timelines.
Such complexity led to patient, caregiver, and researcher fatigue, potentially delaying the delivery of effective cancer prevention and treatment approaches.
Market Opportunity
Diversity Action Plans Opened New Pathways for Oncology Trials
The U.S. FDA’s Diversity Action Plans created a major opportunity for sponsors to design more inclusive oncology trials. These plans required companies to set clear enrolment goals by race, ethnicity, sex, and age, and to explain how they would meet them. This shift broadened the eligible patient base and improved trial relevance across real world populations. The FDA issued draft guidance under FDORA, and by late 2024, several sponsors had already updated protocols and added new community sites to align with diversity expectations.
This policy changed expanded trial networks, reduced recruitment barriers, and improved data generalizability. It is creating opportunities in the oncology clinical trials market by encouraging wider participation and sponsor confidence.
Regional Analysis
The North America region dominated the market with a revenue share of 47.92% in 2025. North America’s market grew strongly because of the integration of electronic health records, biobanks, and population genomics projects that streamlined trial recruitment and monitoring. In the U.S., programs like NIH’s All of Us, which returned genomic results to over 100,000 participants by 2024, expanded trial-ready databases and enhanced personalized trial designs. This integration reduced patient identification barriers, improved trial diversity, and helped sponsors run oncology trials with greater speed and precision.
The U.S. market is anticipated to witness significant market growth owing to the government’s Cancer Moonshot program, which aimed to speed up cancer research by improving access to clinical trials and funding new trial models. In 2024, the NCI expanded community-based trial networks, making oncology studies available in more local and underserved areas. This helped improve trial participation and gave sponsors the chance to test therapies in real-world, diverse patient settings.
Asia Pacific Market Insights
The Asia Pacific region is the fastest-growing region with a CAGR of 9.52% during the forecast timeframe, owing to strong government support for precision medicine programs and heavy investments in genomics infrastructure. Countries like China, Japan, and South Korea improved access and affordability of cancer testing and trial participation. These initiatives encouraged more oncology trials across the region.
The Chinese market is anticipated to witness significant market growth owing to the regulatory flexibility that allows the use of real-world evidence (RWE) to support drug approvals. In 2024, the NMPA approved several oncology drugs using RWE from pilot zones like Hainan Boao. This approach encouraged more sponsors to run oncology trials in China.
Pie Chart: Regional Market Share, 2025
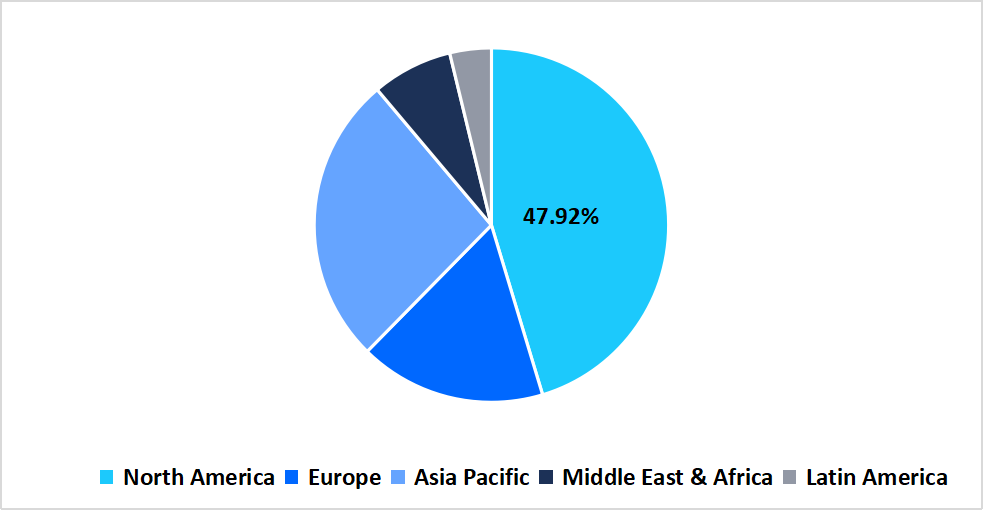
Source: Straits Research
Europe Market Insights
In Europe, the market is witnessing steady growth driven by continuous advancements in healthcare infrastructure and patient care standards. Ongoing updates in diagnostic and treatment protocols across European nations have fostered the adoption of innovative therapeutic approaches. Several cancer centers across the region upgraded their facilities, aligning with new standards of cancer care. These enhancements have accelerated demand for novel oncology drugs and advanced clinical research, thereby propelling the growth of the oncology clinical trials market in Europe.
In Spain, the oncology clinical trials market is expanding significantly, supported by the rising cancer burden across age groups. As shown in the graph, cancer risk increases markedly with age, reaching 47.8% among men and 32.7% among women aged 80–84. This escalating incidence underscores the urgent need for advanced therapeutic interventions and the continued development of innovative oncology treatments through clinical research.
Percent Probability of Cancer Development by Gender & Age in Spain, 2024
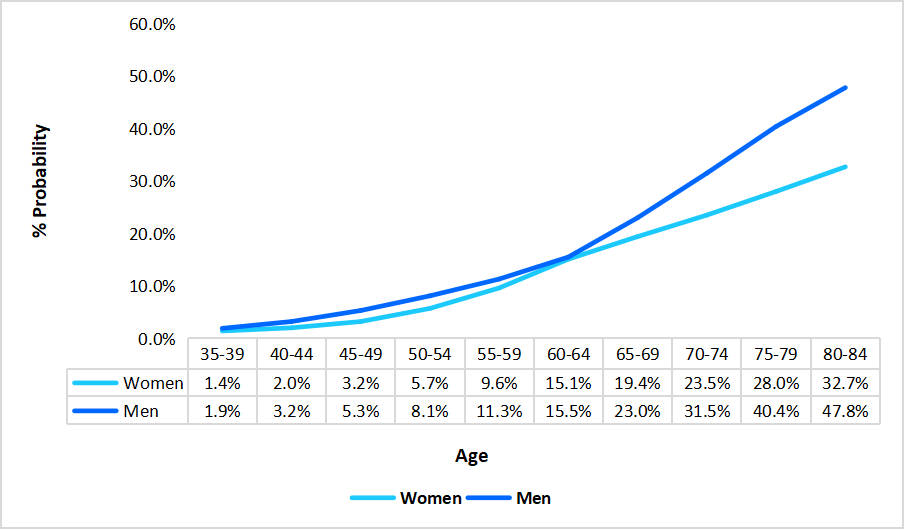
Source: Straits Research
Middle East and Africa Market Insights
The oncology clinical trials market in the Middle East and Africa is witnessing steady growth driven by rapid advancements in cancer care infrastructure and accessibility to oncology drugs. Countries such as Saudi Arabia, the UAE, and South Africa are investing heavily in specialized cancer centers to enhance cancer diagnosis, treatment, and research capabilities.
In South Africa, the oncology clinical trials market is gaining traction with growing initiatives to enhance childhood cancer care. The implementation of the Paediatric Oncology Facility Integrated Local Evolution (ProFILE) tool is enabling systematic evaluation and improvement of pediatric oncology services nationwide. As cancer care delivery becomes more structured, equitable, and accessible, the rising demand for innovative oncology drugs and therapies for childhood cancers is expected to significantly contribute to the growth of South Africa’s market.
Latin America Market Insights
Across Latin America, the market is experiencing strong growth driven by the widespread adoption of national cancer screening initiatives. Countries such as Brazil, Mexico, and Argentina are implementing large-scale programs targeting breast, cervical, and colorectal cancers to enhance early detection and improve treatment outcomes. Government-supported efforts, including Brazil’s Unified Health System (SUS), are expanding access to preventive healthcare and promoting timely interventions, thereby increasing the demand for advanced diagnostics, oncology drugs, and clinical research in the region.
Mexico is emerging as a key hub for oncology clinical trials, supported by major investments from local pharmaceutical companies. The announcement by four Mexican pharmaceutical companies, Kener, Genbio, Neolpharma, and Neolsym, to invest over USD 722 million presents a significant opportunity for the country’s oncology clinical trials market. Amid chronic shortages of essential cancer medications and limited access to innovative therapies, this investment supports the federal government’s Plan México initiative, which aims to boost domestic pharmaceutical production and research capacity. By strengthening local capabilities for drug development and clinical research, these investments accelerated trial initiation, improved patient enrollment, and enhanced the availability of innovative oncology therapies, fostering a more dynamic and resilient clinical trials ecosystem in the country.
Phase Insights
The Phase I segment is dominated the market in 2025 with a revenue share of 35.26%, owing to the increasing focus on early stage drug development, the rising number of novel oncology compounds entering clinical evaluation, and the need to assess safety and dosage in humans.
The Phase III is estimated to register the fastest CAGR of 9.23% during the forecast period, owing to the fact that phase III trials are the costliest as they involve a large number of participants. These trials also include long term safety studies conducted for regulatory approval and post marketing commitments.
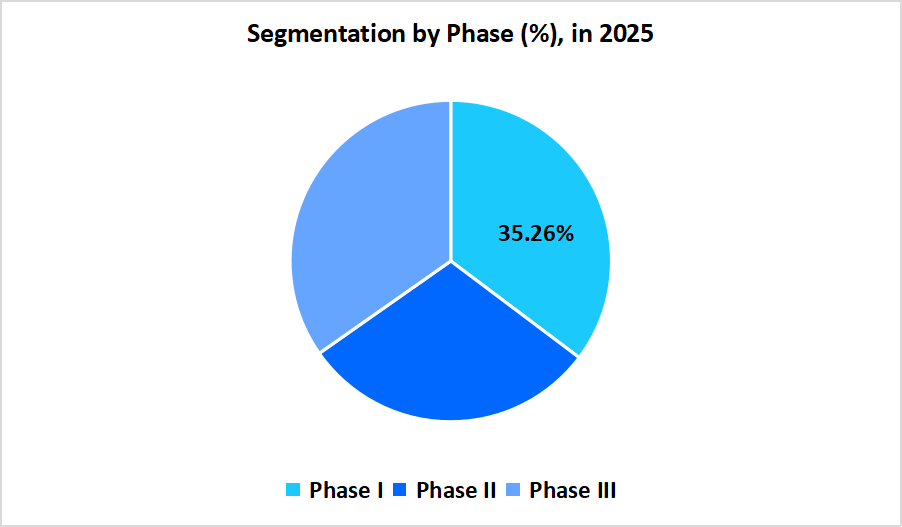
Source: Straits Research
Type Insights
The prostate cancer segment is anticipated to grow at a CAGR of 9.12% during 2026-2034. This growth is attributed to the rising prevalence of prostate cancer among aging populations, increasing awareness and early detection programs, and the development of innovative targeted therapies.
The breast cancer segment dominated the market, with a revenue share of 17.23%, owing to strong focus on early detection and personalized therapies. Continuous advancements in hormone, targeted, and immunotherapies drive extensive research activity. Moreover, substantial funding and awareness initiatives further accelerate clinical development in this segment.
Study Insights
The interventional studies segment dominated the market in 2025, with a revenue share of 75.63%. Interventional studies remained the backbone of oncology trials as they directly tested experimental therapies in controlled settings. Their ability to generate strong evidence on safety and efficacy made them the preferred approach for sponsors and regulators, driving continuous growth and innovation in the oncology clinical trials market.
The observational studies are estimated to register the fastest CAGR during the forecast period, due to the growing emphasis on long-term patient outcome tracking, and post-marketing surveillance. These studies provide valuable insights into treatment effectiveness, safety, and patient quality of life beyond controlled trial settings.
Competitive Landscape
The global market is highly fragmented with the presence of numerous multinational pharmaceutical companies, contract research organizations (CROs), and emerging biotech firms actively engaged in developing innovative therapies.
OncoNova Therapeutics, Inc.: An emerging market player
OncoNova Therapeutics, Inc., is an emerging player in the global market, focusing on novel small-molecule therapeutics targeting hematologic malignancies and solid tumors.
- In April 2025, OncoNova Therapeutics, Inc. initiated Phase II clinical trials for its lead candidate, ONC-123, a targeted therapy designed to inhibit cyclin-dependent kinases in patients with relapsed or refractory acute myeloid leukemia (AML).
List of Key and Emerging Players in Oncology Clinical Trials Market
- Hoffmann-La Roche Ltd
- AstraZeneca
- Merck & Co., Inc.
- Gilead Sciences, Inc
- PARAXEL International Corporation
- ICON plc
- Syneos Health
- Medpace
- Novotech
- Charles River Laboratories
- Laboratory Corporation of America Holdings
- Pfizer Inc.
- Lilly USA, LLC
- Novartis AG
- Bristol-Myers Squibb Company
- AbbVie Inc.
- Amgen Inc.
- Sanofi
- GSK plc.
- Bayer AG
- Others
Strategic Initiatives
- July 2024: Thermo Fisher Scientific Inc. partnered with the National Cancer Institute on the myeloMATCH precision medicine umbrella trial.
- March 2024: Bayer AG and Thermo Fisher Scientific Inc. partnered to develop next-generation sequencing (NGS) based companion diagnostic assays.
- January 2024: Perexel and the Japanese Foundation for Cancer Research collaborated to accelerate access to oncology clinical trials in Japan.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 18.55 billion |
| Market Size in 2026 | USD 20.04 billion |
| Market Size in 2034 | USD 38.23 billion |
| CAGR | 8.41% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Phase, By Type, By Study |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Oncology Clinical Trials Market Segments
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Type
- Breast Cancer
- Melanoma Cancer
- Colorectal Cancer
- Prostate Cancer
- Lung Cancer
- Others
By Study
- Interventional studies
- Observational studies
- Expanded access studies
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































