North America, accounting for 37.6% of the market share, dominates the pharmacogenomics market revenue, owing to its advanced healthcare infrastructure, strong government support for precision medicine, and widespread adoption of genetic testing. The region benefits from a high concentration of key market players, extensive research funding, and integration of pharmacogenomic data into electronic health records (EHRs). Additionally, initiatives such as the U.S. FDA’s Table of Pharmacogenomic Biomarkers in Drug Labeling and the NIH’s All of Us Research Program have accelerated clinical adoption. Favorable reimbursement policies and the presence of leading diagnostic labs further contribute to North America's dominant position in the global market.
Pharmacogenomics (PGX) Market Size, Share & Trends Analysis Report, By Type (Products (Genotyping Kits, Sequencing Instruments, PCR Reagents and Consumables, Sample Preparation Kits and Others), Services (Genetic Testing Services, Pharmacogenomic Consulting and Others) and Software (Bioinformatics Software, Clinical Decision Support System, Laboratory Information Management System, EHR Integration Software and Others)), By Technology (Next-Generation Sequencing, Polymerase Chain Reaction, Mass Spectrometry, Microarray and Others), By Application (Cardiovascular disease, Infectious diseases, Oncology, Neurological diseases, Pain Management and Trauma and Others) (By End User (Hospitals, Clinical Laboratories, Pharmaceutical Companies, Academic Research Institutions and Others), and By Region (North America, Europe, Asia Pacific, Middle East & Africa, Latin America) Forecasts, 2025-2033
Pharmacogenomics (PGX) Market Overview
The global pharmacogenomics (PGX) market size was valued at USD 6.13 billion in 2024 and is projected to grow from USD 6.69 billion in 2025 to USD 11.17 billion by 2033, exhibiting a CAGR of 6.6% during the forecast period (2025-2033). The growth of the market is attributed to the Rising burden of adverse drug reactions and increasing integration of pharmacogenomics in clinical workflows.
Key Market Indicators
- North America dominated the pharmacogenomics industry and accounted for a 37% share in 2024.
- Based on type, the Products segment accounted for the largest market share 47% in 2024.
- Based on technology, the Polymerase Chain Reaction (PCR) segment dominated the pharmacogenomics sector with a market share of about 38.09% in 2024.
- Based on application, the Oncology segment dominated the pharmacogenomics market with a 28% share in 2024, driven by the rising use of pharmacogenomic testing to personalize cancer therapies for improved efficacy and safety.
Market Size & Forecast
- 2024 Market Size: USD 6.13 billion
- 2033 Projected Market Size: USD 11.17 billion
- CAGR (2025–2033): 6.6%
- Asia-Pacific: Largest market in 2024
- North America: Fastest-growing region
Pharmacogenomics is a wide field comprising the study of the genome's reaction to drugs, combining the fields of pharmacology and genomics. It helps to understand how individual genetic differences influence drug efficacy and the adverse events. The major factor propelling the market of pharmacogenomics is the growing interest in personalized and precision medicine. Research and development focusing on every therapeutic area in the pharmaceutical sector has gained significant interest in personalizing care for patients to obtain better drug optimization and fewer adverse events. Medicinal response highly differs from patient to patient and thus results in potential adverse drug reactions.
The National Institute of Health (NIH) reported that more than 6% of hospital admissions are due to adverse reactions to medicines. One such example is simvastatin, a medication prescribed for high cholesterol. Some people have genomic variants in the SLCO1B1 gene that alter the protein and slow this transport, causing the drug to build up in the blood and leading to pain and muscle weakness. Such adverse drug reaction incidences have enhanced the traction towards the pharmacogenomics field. The pharmacogenomics studies have significantly increased over the last decade, highlighting one of the emerging fields of the healthcare industry. The rapid increase in epidemiologic, translational, and implementation studies has resulted in the formulation of guidelines and increased usage of pharmacogenomics in clinical practice. The graph below represents the rapid increase in the pharmacogenomic implementation studies over the last decade.
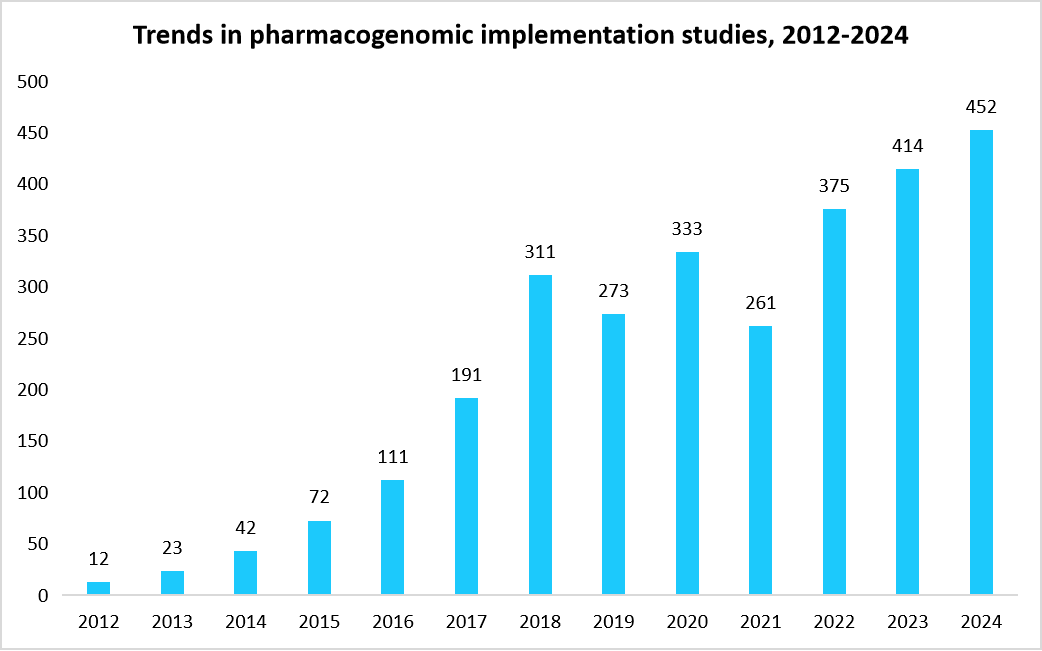
Source: Centers for Disease Control and Prevention and Straits Analysis
This reflects a significant increase in researchers' focus on understanding the effects of drugs on an individual’s genetic profile. Additionally, the pharmacogenomics market is witnessing significant innovations in the field of genetic testing, particularly in high-throughput genotyping, next-generation sequencing (NGS), and bioinformatics platforms that enable rapid and cost-effective genomic analysis. Such innovations are further accelerating the clinical adoption of pharmacogenomic testing across numerous therapeutic areas, such as oncology, psychiatry, cardiology, neurology, and infectious diseases.
However, high testing costs, particularly genomic testing for chronic diseases, and limited availability of reimbursements for genomic testing impede the market growth, lowering the adoption of pharmacogenomics among patients.
On the other hand, the development of bioinformatics and data analytics tools region-specific or indication-specific for pharmacogenomic studies and expanding application in mental health and psychiatry presents significant opportunities in the market for pharmacogenomics, opening new avenues for patient-driven healthcare. Emerging markets with growing investments in genomics infrastructure, along with government-led precision medicine initiatives, offer untapped potential for market penetration and strategic collaborations.
Pharmacogenomics Market Trends
Integration of Ai and Machine Learning-Driven Bioinformatics Tools
Interpretation of vast genomic data is usually complex and exhaustive; however, growing integration of AI and machine learning driven tools in pharmacogenomics datasets has improved clinical decision-making by reducing manual interpretation time and accuracy.
- For instance, companies such as Deep Genomics and Tempus use AI platforms to match patients with genetically optimized therapies, accelerating precision medicine adoption.
- Additionally, the U.S. National Institutes of Health (NIH) has recognized this shift, investing in initiatives such as the Bridge2AI program, which supports the use of AI in analyzing biomedical data, including genomics.
- Further, the NIH’s All of Us Research Program, which aims to collect genetic data from over one million Americans, increasingly relies on machine learning for data interpretation and personalized treatment modelling.
As healthcare systems prioritize efficiency and scalability in pharmacogenomic studies, AI-powered tools are becoming integral to pharmacogenomic testing workflows, marking a clear transition toward tech-enabled, data-driven personalized care, boosting the market growth.
Rising Focus on Preventive Care
Rising focus on preventive care is enhancing the adoption of pharmacogenomics across the healthcare setting, as physicians, by analysing genetic variations which will influence drug metabolism, aim to reduce adverse drug reactions and focus on improving long-term treatment outcomes. This will increase the need for pharmacogenomic testing to support proactive prescribing and safer medication management. The Food and Drug Administration (FDA) has mandated manufacturers to include labelling instructions for genetic profiles.
- For instance, as of September 2024, over 500 medications now include pharmacogenomic information on their label, demonstrating the growing integration of genomics into preventive strategies.
In addition, the government's focus on the preventive care model boosts the need for pharmacogenomic testing,
- For instance, the PHASER (Pharmacogenomics Action for Cancer Survivorship) program is a collaboration between the U.S. Department of Veterans Affairs and NIH, aimed at bringing pre-emptive pharmacogenomic testing to veterans nationwide. It seeks to integrate pharmacogenomics data into care plans before prescribing medications, especially in cancer survivors, aligning with preventive care principles.
Thus, this shift toward a preventive care model boosts the market growth, reflecting pharmacogenomics as a critical pillar of modern preventive medicine.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 6.13 Billion |
| Estimated 2025 Value | USD 6.69 Billion |
| Projected 2033 Value | USD 11.17 Billion |
| CAGR (2025-2033) | 6.6% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | QIAGEN, Illumina, Inc., Agilent Technologies, Inc., Foundation Medicine, Inc., Thermo Fisher Scientific Inc. |

to learn more about this report Download Free Sample Report
Pharmacogenomics Market Growth Factors
Rising Burden of Adverse Drug Reactions
Adverse drug reactions are one of the major public health concerns, resulting in increased hospital admissions. For instance,
- U.S. Centers for Disease Control and Prevention in April 2024, reported that more than 1.5 million people visit the emergency department for adverse drug reactions each year in the U.S.
- In addition, 1,522 adverse drug reactions were reported in the period of January to December 2023 in only Abu Dhabi as reported by the Department of Health Abu Dhabi. Further, the graph below represents continuously increasing adverse drug reactions in the last decade across the country.
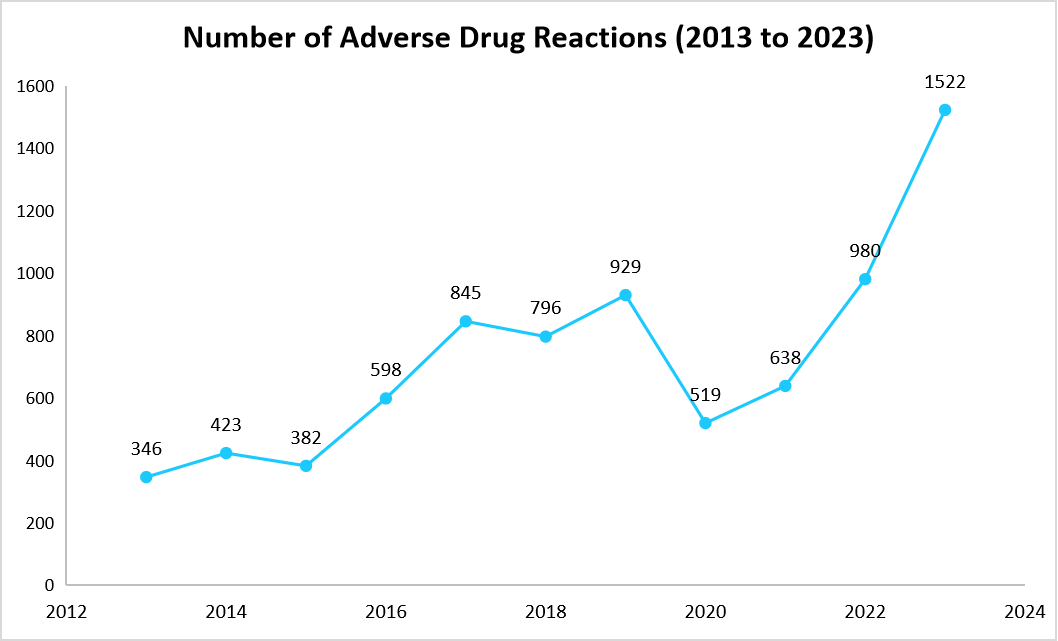
Source: Department of Health, Abu Dhabi, and Straits Research
Pharmacogenomics plays a significant role in mitigating these risks by identifying genetic variations that influence how an individual’s genetic profile responds to medications. As the demand for safer and more effective treatments grows, pharmacogenomic testing is gaining widespread attention as a preventive strategy integrated into prescribing practices.
Increasing Integration of Pharmacogenomics in Clinical Workflows
Pharmacogenomics is increasingly integrated into the routine clinical workflow, particularly in hospitals and primary care settings, to personalize treatment regimens and improve patient safety. For instance,
- The Mayo Clinic has implemented pharmacogenomics testing into its clinical workflows through its RIGHT (Right Drug, Right Dose, Right Time) program, which proactively genotypes patients and stores their results in the EHR for use during future prescriptions.
- Further, in 2024, the U.S. National Human Genome Research Institute (NHGRI) reported that over 75 healthcare systems in the U.S. have begun incorporating pharmacogenomics data into clinical decision-making tools, reflecting a growing standardization of genomic-guided care.
Such integration enables physicians to utilize a patient’s genetic information at the point of care, thereby reducing trial-and-error prescribing and optimizing drug efficacy. Major healthcare systems are now adopting decision-support tools that incorporate pharmacogenomics data into electronic health records (EHRs), allowing real-time access to genotype-guided prescribing recommendations, thus driving the market growth.
Market Restraining Factor
Ethical, Legal, and Social Considerations for Pharmacogenomics
Pharmacogenomics study highly lacks population diversity in genomic research, limiting the adoption of pharmacogenomics. For instance,
- NIH reported that the genomic data used to develop pharmacogenomic tests are usually not representative of diverse populations, and are often based on European Ancestry. This highlights the lack of genomic variants that are more common in some populations.
- Further, the U.S. National Institutes of Health (NIH) reported, over 75% of participants in genome-wide association studies (GWAS) were of European descent in 2024, highlighting a major gap in equitable pharmacogenomics development.
Such incidents lead to inequities in treatment outcomes and limit the development of effective therapies for globally diverse populations. Moreover, underrepresentation hampers the clinical translation of pharmacogenomics across global healthcare systems, limiting the market growth.
Market Opportunity
Development of Population-Specific Pharmacogenomic Panels
Genetic diversity among the population has increased the focus on the development of population-specific pharmacogenomic panels. These panels are specifically designed for the genetic variants that are prevalent in specific ethnic or regional groups, improving drug efficacy and safety across underrepresented populations. Such tailored panels allow for more accurate predictions of drug response, helping healthcare providers minimize adverse drug reactions and optimize dosage based on the patient's ancestry.
- For instance, HLA-B*15:02, a variant associated with severe skin reactions to carbamazepine, is more prevalent in Southeast Asian populations, prompting region-specific screening guidelines in countries such as Thailand and Singapore. This has resulted in increased awareness of genetic diversity, creating a need for the development of population-specific panels.
- Further, government support to establish national pharmacogenomics programs across the countries to prevent chronic conditions such as Stevens–Johnson Syndrome and toxic epidermal necrolysis, which are highly region-specific, has further increased the need for population-specific panels.
This opens up vast opportunities for pharmacogenomics companies to innovate and capture new market segments, especially in population-specific pharmacogenomics studies.
Regional Analysis
U.s. Pharmacogenomics Market Trends
The U.S. leads the pharmacogenomics landscape, driven by widespread genetic variant prevalence and integration into routine care. According to NHGRI, more than 98% of individuals have genomic variants that can influence drug response, underscoring the broad applicability of pharmacogenomics testing. In 2022, according to the NIH factsheet for pharmacogenomics 14% of FDA-approved medications included pharmacogenomic testing recommendations, potentially impacting 6.7 billion outpatient prescriptions in the U.S. This federal-level acknowledgment highlights how pharmacogenomics is becoming integral to prescribing practices. Through its robust genomics infrastructure and regulatory backing, the U.S. is setting the standard for integrating pharmacogenomic insights into preventive, personalized healthcare.
Asia Pacific Pharmacogenomics Market Trends
The Asia Pacific region is anticipated to grow at the fastest CAGR during the forecast period. This growth is driven by increasing investments in genomic research, expanding healthcare infrastructure, and a rising focus on precision medicine across emerging economies. Countries such as China, Japan, South Korea, and India have launched national genome initiatives to map genetic diversity and enhance personalized treatment.
- For instance, China’s Precision Medicine Initiative, with an estimated funding of USD 9.2 billion by 2030, aims to integrate genomics into routine care. Moreover, Japan’s Tohoku Medical Megabank Project and India’s GenomeIndia initiative support the development of population-specific pharmacogenomic data, fueling regional growth.
China is rapidly adopting pharmacogenetic testing, particularly in hypertension management within its primary healthcare system. A 2023 report from the Chinese Center for Disease Control and Prevention noted that about 270 million adults in China have hypertension, with blood pressure response partly determined by genetics. As a result, pharmacogenomic screening programs such as China‑PEM have been launched, and many hospitalsincluding Fuwai and Peking University First Hospital, are integrating pharmacogenomics tests into standard care. Additionally, China’s national public health policies (2017–2025) actively promote the use of pharmacogenomics in medication safety and rational drug use. These coordinated efforts mark China as a rising leader in applying pharmacogenomics for preventive and population-scale healthcare.
India is advancing pharmacogenomics through its flagship GenomeIndia Project, funded by the Department of Biotechnology. By February 2024, the initiative had sequenced 10,074 genomes across 99 ethnic communities, creating a robust reference for genetic diversity and pharmacogenomic insight that uncovered over 135 million genetic variants, including 7 million novel ones with potential clinical relevance for drug response. Pioneers such as Dr. Vinod Scaria are leveraging this data to develop nationwide pharmacogenomic mapping and integrate findings into precision treatment models. India’s extensive genomic database now forms a critical foundation for population-specific drug guidance and preventive care frameworks.
Europe Pharmacogenomics Market Trends
The UK is rapidly advancing pharmacogenomics through coordinated NHS initiatives and strong public support. In 2024, NHS England launched the Pilot Network of Excellence for Pharmacogenomics, uniting national task forces to develop pan-UK genomic test directories and regulatory frameworks. Public acceptance is high; nearly 90% of adults surveyed indicated they would agree to genetic testing to tailor medication use, and 85% stated the NHS should offer pharmacogenomic testing to patients with multiple conditions. This combination of institutional momentum and public readiness positions the UK as a global model for integrating pharmacogenomics into routine healthcare.
France has launched the ambitious Plan France Médecine Génomique 2025 (PFMG2025), committing USD 263.77 million toward integrating genomic medicine, including pharmacogenomics, into routine clinical care by the end of 2023. Under this national initiative, 12,737 genomic test results were delivered for rare disease and cancer patients by late 2023, with a diagnostic yield of approximately 31% and median turnaround times of 202 days for rare diseases and 45 days for cancer. This large-scale public funding and implementation momentum highlight France's commitment to embedding pharmacogenomics into precision healthcare.
South Africa’s remarkable genetic diversity offers significant opportunities for pharmacogenomics. The study conducted at Steve Biko Academic Hospital showed that proactive pharmacogenomics-guided prescriptions could reduce clinically relevant adverse drug reactions by around 30%. Additionally, the Sydney Brenner Institute is building a biobank with over 17,000 African samples and more than 200 whole genomes, supporting advanced pharmacogenomic research. These initiatives underscore South Africa's growing capacity to leverage population-specific genetic data, positioning its healthcare system to advance personalized medicine and improve treatment outcomes at scale.
Brazil is pioneering pharmacogenomics through the INCA pharmacogenomic program, funded by the Brazilian Ministry of Health. This initiative implemented pharmacogenomics testing for fluoropyrimidines, irinotecan, and thiopurines in cancer patients, covering 100 gastrointestinal and 162 pediatric leukaemia patients, and expanded to three additional oncology centers. Standard operating procedures were established for sample processing, genotyping (DPYD, UGT1A1, TPMT, NUDT15), and EHR integration, achieving an average 10-day turnaround for dosing recommendations. About 10% of patients required adjusted dosing based on their genetic profiles. This public institution’s success demonstrates Brazil's capacity to deliver proactive pharmacogenomic-driven care and serves as a model for scaling personalized treatment across national health services.
Market Segmentation
Type Insights
The market is segmented into products, services, and software. Further, the product segment is sub-segmented into genotyping kits, sequencing instruments, PCR reagents and consumables, sample preparation kits, and others. The services segment is further sub-segmented into genetic testing services, pharmacogenomic consulting, and others. And software segment is sub-segmented into bioinformatics software, clinical decision support systems, laboratory information management systems, EHR integration software, and others.
The products segment accounted for the largest pharmacogenomics market share, owing to their widespread use in both clinical and research settings, where they serve as the primary tool for identifying gene-drug interactions. Particularly, genotyping kits are increasingly being used in routine diagnostics, enabling clinicians to predict adverse drug reactions and optimize drug selection. For instance, according to the U.S. National Library of Medicine's Pharmacogenomics Knowledgebase 2024, CYP2C19, CYP2D6, and SLCO1B1 genotyping, typically conducted using these kits that are now part of standard care in several hospitals across North America and Europe. Further, their compatibility with existing lab infrastructure makes them highly preferred over complex sequencing platforms in standard pharmacogenomics workflows.
Technology Insights
The market is segmented into next-generation sequencing, polymerase chain reaction, mass spectrometry, microarray, and others. The polymerase chain reaction segment dominated the pharmacogenomics sector. This growth is driven due to its high sensitivity, specificity, cost-effectiveness, and widespread adoption in clinical settings for genotyping drug-metabolizing enzyme variants. Additionally, the Centers for Disease Control and Prevention has reported polymerase chain reaction as “widely used in genotyping,” demonstrating how foundational they are to pharmacogenomic applications. Further, this technology, due to its strong clinical relevance, established adoption, and cost efficiency, is driving the segment growth in the pharmacogenomics market.
Application Insights
The market is segmented into cardiovascular disease, infectious diseases, oncology, neurological diseases, pain management and trauma, and others. The oncology segment is anticipated to register the fastest CAGR during the forecast period, owing to increasing use of pharmacogenomic testing to tailor cancer therapies based on individual genetic profiles, improving both efficacy and safety of treatment. Further, the U.S. FDA has approved numerous drugs in oncology that require or recommend companion diagnostic testing, such as KRAS, EGFR, TPMT, and DPYD genotyping, reinforcing pharmacogenomics as standard care in cancer treatment. For instance, according to the U.S. Food and Drug Administration (FDA), as of 2024, over 75% of pharmacogenomic drug labels that include genetic biomarker information are related to oncology therapies. This includes drugs for lung, breast, colorectal, and hematologic cancers. Further, cancer therapies, particularly targeted treatments and chemotherapies, are highly sensitive to genetic variations, further driving the segment growth.
End User Insights
The market is segmented into hospitals, clinical laboratories, pharmaceutical companies, academic research institutions, and others. The clinical laboratories segment is anticipated to register the fastest CAGR during the forecast period, owing to increasing demand for decentralized and rapid genetic testing services, coupled with the expansion of molecular diagnostics infrastructure across developed and emerging economies. Additionally, clinical labs are increasingly becoming the first point of contact for pharmacogenomic testing, driven by physician referrals, insurance coverage, and an expanding test portfolio. Further, the shift from centralized hospital testing to standalone or networked clinical labs allows for quicker turnaround times and greater accessibility, especially in outpatient and community care settings, and is further fuelling the segment growth in the forecast period.
Company Market Share
Companies in the industry are focusing on adopting key business strategies, such as product launches, acquisitions, and product approvals, to gain a strong foothold in the market.
Pgxai: An Emerging Company in the Pharmacogenomics Market
PGxAI is a cutting-edge precision medicine startup that integrates AI and multi-omics to drive real-time pharmacogenomic decision-making.
Recent developments by PGxAI:
- In June 2025, PGxAI launched Betelgeuse, a real-time AI model that adapts treatment recommendations using multi-omics and patient data, and can reduce adverse event risk by approximately 30%.
List of Key and Emerging Players in Pharmacogenomics (PGX) Market
- QIAGEN
- Illumina, Inc.
- Agilent Technologies, Inc.
- Foundation Medicine, Inc.
- Thermo Fisher Scientific Inc.
- Admera Health
- Myriad Genetics, Inc.
- Beckman Coulter, Inc.
- OneOme
- Genomind
- Coriell Life Sciences
- PGxAI
- Tempus
- DEEP GENOMICS
- Helix, Inc.
- Oxford Nanopore Technologies plc.
Recent Developments
- August 2025: The FDA approved the once-weekly subcutaneous injection of Leqembi (lecanemab-irmb) for maintenance dosing to treat early Alzheimer's disease. This is a personalized therapy where genetic factors, specifically ApoE e4 carriers, are considered.
- June 2025: Agilent Technologies introduced its new Hybrid Multisampler and LC Single Quadrupole Mass Spectrometers at the HPLC 2025 conference. These products are designed to enhance analytical performance for therapeutic peptides and proteins, which is relevant for drug discovery and development.
- May 2025: Thermo Fisher Scientific highlighted its innovative and comprehensive solutions for human genetics at the European Society for Human Genetics (ESHG) 2025 conference. The company showcased workflows for genotyping, gene expression, and liquid biopsy analysis, which are key components of pharmacogenomic research and applications.
- March 2025: Beckman Coulter announced FDA clearance for its DxC 500i Clinical Analyzer, an integrated system for clinical chemistry and immunoassay testing. While not exclusively a PGx product, this development expands the company's diagnostic portfolio for clinical use.
Analyst Opinion
The pharmacogenomics market is experiencing robust growth globally, driven by growing focus on personalized treatments. Additionally, expanding direct-to-consumer genetic testing and integration of pharmacogenomics in clinical workflows further propels the demand for pharmacogenomic tools. In addition, integration of advanced analytical tools in pharmacogenomics will enhance the efficacy and turnaround time for genomic analysis, increasing the adoption of pharmacogenomics in healthcare settings. Moreover, government and FDA implementation of various programs to promote genomic testing for pharmaceutical drugs will propel the market growth. However, the high cost and ethical, legal and social concerns of genetic testing limit the adoption of pharmacogenomics in emerging markets. Despite these concerns, development and advancement in pharmacogenomic tools will open new avenues creating significant opportunities in the market.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 6.13 Billion |
| Market Size in 2025 | USD 6.69 Billion |
| Market Size in 2033 | USD 11.17 Billion |
| CAGR | 6.6% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Technology, By Application, By End-User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Pharmacogenomics (PGX) Market Segments
By Type
-
Products
- Genotyping Kits
- Sequencing Instruments
- PCR Reagents and Consumables
- Sample Preparation Kits
- Others
-
Services
- Genetic Testing Services
- Pharmacogenomic Consulting
- Others
-
Software
- Bioinformatics Software
- Clinical Decision Support System
- Laboratory Information Management System
- EHR Integration Software
- Others
By Technology
- Next-Generation Sequencing
- Polymerase Chain Reaction
- Mass Spectrometry
- Microarray
- Others
By Application
- Cardiovascular disease
- Infectious diseases
- Oncology
- Neurological diseases
- Pain Management and Trauma
- Others
By End-User
- Hospitals
- Clinical Laboratories
- Pharmaceutical Companies
- Academic Research Institutions
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Mitiksha Koul
Research Associate
Mitiksha Koul is a Research Associate with 2 years of experience in market research. She focuses on analyzing industry trends, competitive landscapes, and growth opportunities to support strategic decision-making. Mitiksha’s strong analytical skills and research expertise enable her to deliver actionable insights that help businesses adapt to evolving market dynamics and achieve sustainable growth.










































