Preeclampsia Diagnostics Market Size, Share & Trends Analysis Report By Product Type (Instrument, Consumables), By Test (Blood Test, Urine Test), By End-User (Hospitals, Specialty Clinics, Diagnostic Centers, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Preeclampsia Diagnostics Market Overview
The global preeclampsia diagnostics market size is estimated at USD 1.08 billion in 2025 and is projected to reach USD 1.46 billion by 2034, growing at a CAGR of 3.44% during the forecast period. Remarkable growth of the market is propelled by the rising adoption of biomarker-based testing for early risk prediction, the increasing incorporation of automated immunoassay systems in hospital laboratories, and growing clinical awareness about the importance of early detection in reducing maternal morbidity.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 40.11% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 5.44%.
- Based on Product Type, the instrument segment is projected to record the fastest growth of 4.64% over the forecast period.
- Based on the Test, the urine test segment is anticipated to grow at the fastest CAGR of 4.34%.
- Based on End User, the hospitals segment dominated the market in 2025 with a revenue share of 37.28%.
- The U.S. dominates the global market, valued at USD 398.55 million in 2024 and reaching USD 410.83 million in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
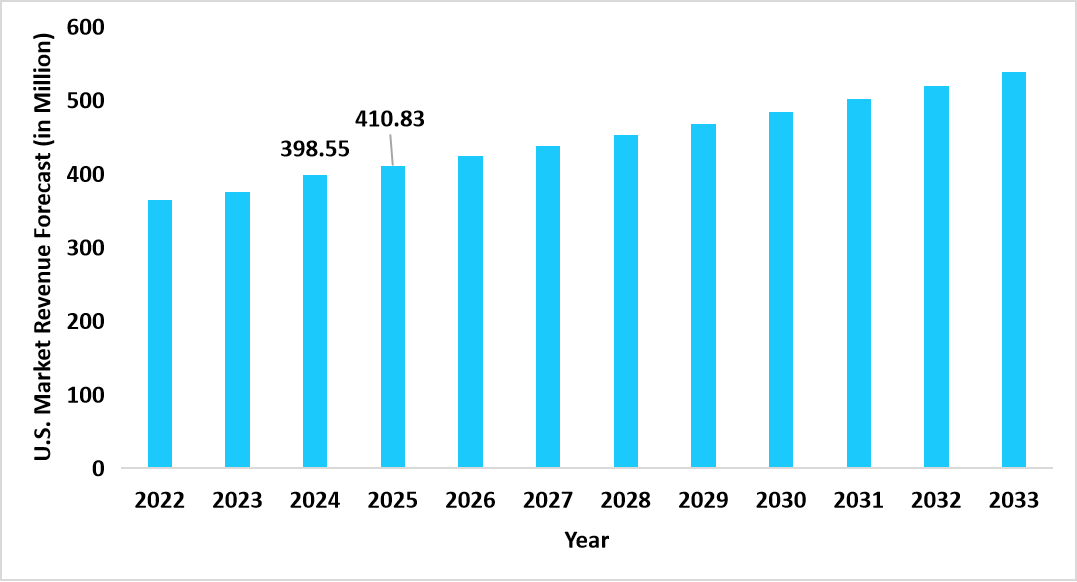
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.08 billion
- 2034 Projected Market Size: USD 1.46 billion
- CAGR (2025 to 2034): 3.44%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The preeclampsia diagnostics market refers to the industry focused on the development and commercialization of instruments, consumables, and testing solutions used to detect and monitor preeclampsia, a pregnancy-related hypertensive disorder that can lead to serious maternal and fetal complications if left untreated. The market is segmented by product type into instruments, including immunoassay analyzers, point-of-care devices, and laboratory systems, and consumables such as reagents, assay kits, and calibration materials used for biomarker-based analysis. Based on test type, it comprises blood tests that measure angiogenic biomarkers like sFlt-1 and PlGF for early detection, and urine tests that evaluate protein levels associated with hypertensive conditions in pregnancy. By end user, the market serves hospitals, specialty clinics, diagnostic centers, and other healthcare facilities where these diagnostic tools are integrated into prenatal care workflows to support timely diagnosis, risk stratification, and improved clinical management of pregnant women at risk of developing preeclampsia.
Latest Market Trends
Integration of Artificial Intelligence and Predictive Analytics in Risk Assessment
A key trend in the preeclampsia diagnostics market is the integration of artificial intelligence and predictive analytics for early risk prediction and clinical decision-making. Advanced AI models are being incorporated into diagnostic platforms to analyze maternal biomarkers, blood pressure patterns, and patient history, allowing clinicians to identify high-risk pregnancies at earlier stages. Predictive analytics enhances accuracy in disease stratification, minimizes false positives, and supports tailored monitoring strategies that improve prenatal outcomes across different care settings.
Adoption of Biomarker-Based Point-of-Care Testing
The growing use of biomarker-based point-of-care testing is transforming preeclampsia diagnostics by enabling rapid detection of protein ratios such as sFlt-1/PlGF directly at the patient’s bedside. Healthcare providers are increasingly implementing portable analyzers that deliver immediate results, reducing reliance on centralized laboratories. This trend supports faster clinical decision-making, improves access to timely screening in rural or low-resource areas, and aligns with the global shift toward decentralized maternal health diagnostics.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.08 Billion |
| Estimated 2026 Value | USD 1.11 Billion |
| Projected 2034 Value | USD 1.46 Billion |
| CAGR (2026-2034) | 3.44% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Abbott, Hoffmann-La Roche Ltd, Thermo Fisher Scientific Inc. , Revvity Inc., Diabetomics, Inc. |
to learn more about this report Download Free Sample Report
Preeclampsia Diagnostics Market Drivers
Rising Focus on Early Detection to Prevent Maternal and Fetal Complications
The increasing emphasis on early and accurate detection of preeclampsia is driving the demand for advanced diagnostic tools. The condition remains a leading cause of maternal and neonatal mortality, prompting healthcare systems to prioritize routine screening and risk-based assessment. Growing adoption of biomarker-based tests and government-supported maternal health programs is accelerating the implementation of standardized diagnostic protocols, supporting better pregnancy management and reducing hospital burden associated with late-stage complications.
Market Restraints
High Cost and Limited Accessibility of Advanced Diagnostic Technologies
The high cost of specialized biomarker assays and diagnostic instruments restricts widespread adoption, especially in low- and middle-income countries. Limited reimbursement frameworks and the absence of affordable testing infrastructure further constrain market growth. These factors create disparities in diagnostic access, delaying early intervention and limiting the reach of advanced screening programs in under-resourced healthcare systems.
Market Opportunity
Integration of Preeclampsia Testing within Comprehensive Prenatal Screening Programs
Expanding preeclampsia diagnostics within integrated prenatal screening programs presents a promising growth opportunity. Health systems are increasingly combining tests for gestational diabetes, fetal anomalies, and preeclampsia into unified maternal care frameworks. This integration enhances patient convenience, supports early risk management, and encourages widespread screening adoption. The approach also opens pathways for partnerships between diagnostic companies and healthcare providers to deliver cost-efficient, multi-condition maternal testing solutions globally.
Regional Analysis
North America held the largest share of 40.11% in the preeclampsia diagnostics market in 2025, driven by the growing integration of biomarker-based assays and automated screening platforms in hospital maternity units. The adoption of quantitative tests such as the sFlt-1/PlGF ratio has enhanced early risk stratification and clinical decision-making across tertiary care settings. Strong regulatory support and the presence of advanced clinical laboratories are accelerating the standardization of diagnostic protocols for hypertensive disorders during pregnancy.
The U.S. market is influenced by nationwide efforts to reduce maternal morbidity through expanded use of laboratory biomarkers and digital monitoring tools. The Centers for Disease Control and Prevention (CDC) and the American College of Obstetricians and Gynecologists (ACOG) are encouraging the use of validated assays in prenatal screening programs. The integration of electronic health records with diagnostic workflows is allowing obstetricians to interpret test results in real time, improving maternal surveillance and treatment outcomes.
Asia Pacific Market Insights
The Asia Pacific region is the fastest-growing market with a CAGR of 5.44% during the forecast period, supported by government-led prenatal screening initiatives and increased adoption of point-of-care diagnostic systems across hospitals. Expanding access to laboratory services and growing awareness among obstetricians about the benefits of early detection are boosting demand for biochemical testing. Medical institutions in countries like Japan, China, and South Korea are introducing multi-marker panels to improve predictive accuracy in high-risk pregnancies.
The Indian preeclampsia diagnostics market is evolving with the digital expansion of prenatal care under the Ayushman Bharat Digital Mission. Integration of laboratory information systems with national health databases is improving continuity of maternal care. Collaborations between public hospitals, private diagnostic chains, and academic research institutions are fostering localized development of cost-efficient test kits suited to Indian clinical needs.
Pie Chart: Regional Market Share, 2025

Source: Straits Research
Europe Market Insights
The Europe market is expanding due to growing emphasis on structured antenatal care and the inclusion of validated biomarkers in national pregnancy screening guidelines. Strong support from regulatory authorities and public health bodies for standardizing preeclampsia diagnostics is enhancing adoption across hospitals and private clinics. The European Medicines Agency and regional maternal health organizations are facilitating cross-country validation of diagnostic assays for better risk assessment.
Germany’s market is advancing with the implementation of the Digital Healthcare Act, which enables physicians to prescribe approved digital health applications linked with diagnostic testing. Hospitals are increasingly combining laboratory biomarkers with digital tracking tools for continuous monitoring of pregnant women at elevated risk, improving early intervention strategies and clinical decision accuracy.
Middle East and Africa Market Insights
The Middle East and Africa market is experiencing expansion due to rising investments in maternal health infrastructure and broader access to clinical laboratories equipped for biomarker testing. Governments are strengthening tele prenatal monitoring programs to extend diagnostic coverage to remote areas. Integration of mobile health technologies with laboratory platforms is improving prenatal risk evaluation and data sharing among care providers.
The UAE market is growing as the Ministry of Health and Prevention encourages the use of digital health platforms and laboratory-based diagnostic protocols for preeclampsia detection. Hospitals are deploying connected systems that allow real-time sharing of test results between obstetricians and laboratory specialists, leading to improved maternal safety and streamlined clinical workflows.
Latin America Market Insights
The Latin American market is expanding as public health agencies focus on structured antenatal testing and enhanced maternal care programs. Increasing adoption of blood-based biomarker assays and improved laboratory infrastructure across Brazil, Mexico, and Chile are driving growth. Awareness initiatives highlighting the role of early diagnosis in preventing adverse pregnancy outcomes are further strengthening market adoption.
Brazil’s market growth is supported by the national e-health strategy, which promotes integration of prenatal diagnostics with digital health records. Collaboration between healthcare providers and diagnostic laboratories has accelerated the deployment of placental biomarker testing across both public and private maternity hospitals, enhancing early identification and management of at-risk pregnancies.
Product Type Insights
The consumables segment dominated the preeclampsia diagnostics market in 2025, driven by the growing demand for assay kits, reagents, and biomarker panels used in routine testing procedures. Continuous utilization of consumables across both hospital and diagnostic laboratory settings supports recurring revenue streams and ensures consistent product turnover. The rising frequency of maternal health screenings and the adoption of biochemical marker-based testing protocols further reinforced the dominance of this segment.
The instruments segment is projected to record the fastest growth of 4.64% over the forecast period, owing to technological advancements in automated analyzers and portable testing equipment. The introduction of compact systems that deliver high-throughput results and integrate seamlessly with digital monitoring platforms is accelerating adoption, particularly in decentralized diagnostic settings.
Test Insights
The blood test segment held the largest market share in 2025, attributed to its central role in detecting key biomarkers such as sFlt-1, PlGF, and LDH. Blood-based assays remain a standard component of clinical evaluation due to their accuracy in diagnosing disease severity and progression. Increasing adoption of advanced immunoassay platforms and continuous improvements in biochemical analysis are supporting the segment’s sustained growth.
The urine test segment is anticipated to grow at the fastest CAGR of 4.34% during the forecast period. The ease of sample collection and low-cost testing make urine-based diagnostics ideal for early screening, especially in primary care and rural healthcare facilities. The development of dipstick and rapid test formats that provide quick results without specialized equipment is further boosting segment expansion.
End-User Insights
Hospitals segment dominated the market in 2025 with a revenue share of 37.28%, as they represent the primary centers for comprehensive maternal care and high-risk pregnancy management. Large patient inflow, integration of multidisciplinary teams, and access to advanced laboratory infrastructure have supported the higher adoption of diagnostic solutions within hospital environments.
The diagnostic centers segment is forecasted to witness the fastest growth of 4.12% during the projected period. Increasing outsourcing of maternal health testing by hospitals and the expansion of standalone laboratories offering biomarker-based preeclampsia screening are fueling segment growth. The emphasis on fast turnaround times and accessible testing services is further strengthening the role of diagnostic centers in this market.
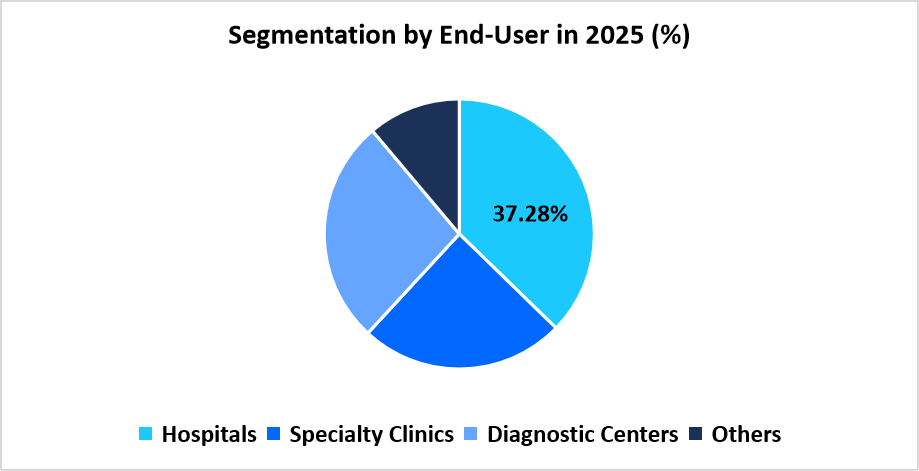
Source: Straits Research
Competitive Landscape
The global preeclampsia diagnostics market is moderately consolidated, with participation from established diagnostic manufacturers, biotechnology firms, and emerging companies focusing on biomarker-based detection and point-of-care screening.
Hoffmann-La Roche Ltd.: An emerging market player
Hoffmann-La Roche Ltd. is a prominent player in the global market, offering advanced biochemical testing solutions designed for early risk assessment of hypertensive disorders during pregnancy.
- In February 2025, Roche announced that its Elecsys sFlt-1/PlGF ratio test received U.S. FDA 510(k) clearance, expanding access to validated diagnostic tools for maternal risk evaluation. The Elecsys assays, designed for use on the cobas e immunoassay analyzers, enable clinicians to identify women at risk of developing preeclampsia within a short predictive window, supporting improved clinical decision-making in antenatal care. This regulatory milestone reinforces Roche’s global leadership in maternal health diagnostics and enhances its presence across hospital-based testing environments.
List of Key and Emerging Players in Preeclampsia Diagnostics Market
- Abbott
- Hoffmann-La Roche Ltd
- Thermo Fisher Scientific Inc.
- Revvity Inc.
- Diabetomics, Inc.
- QuidelOrtho Corporation
- Siemens Healthineers AG
- Bio-Rad Laboratories, Inc.
- BD
- Danaher Corporation
- EKF Diagnostics Holdings plc
- Sera
- NX Prenatal Inc.
- Metabolomic Diagnostics
- Akonni Biosystems, Inc.
- Mirvie, Inc.
- Trinity Biotech plc
- Others
Strategic Initiatives
- October 2025: Mirvie, Inc. reported clinical results for its novel, non-invasive blood test designed to assess a woman’s risk of developing preeclampsia during pregnancy. The test evaluated molecular signatures in maternal blood to detect early risk factors prior to the onset of symptoms, enabling clinicians to implement preventive interventions and personalized monitoring strategies. This advancement highlighted the growing application of precision diagnostics in maternal health, emphasizing a shift toward proactive and predictive prenatal care models for preeclampsia management.
- August 2025: Trinity Biotech plc launched its FDA-cleared PreClara Ratio sFlt-1/PlGF test in collaboration with Thermo Fisher Scientific as a reference laboratory service in the United States. The test provided clinicians with biomarker-based risk stratification for hypertensive disorders of pregnancy, supporting earlier and more informed clinical decisions. Through this partnership, Trinity Biotech leveraged its Immco Diagnostics reference laboratory and commercial network to expand access to an FDA-cleared diagnostic solution in the U.S. maternal health market.
- September 2024: Trinity Biotech completed the acquisition of Metabolomic Diagnostics, the developer of the PrePsia first-trimester predictive test for preeclampsia, for an undisclosed enterprise value. The acquisition provided Trinity with proprietary mass spectrometry and machine learning technologies to advance early-stage risk prediction capabilities. The company announced plans to commercialize the PrePsia test through its Immco Diagnostics laboratory in the United States and to pursue international market expansion, with initial revenue generation expected in 2025.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.08 Billion |
| Market Size in 2026 | USD 1.11 Billion |
| Market Size in 2034 | USD 1.46 Billion |
| CAGR | 3.44% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product Type, By Test, By End-User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Preeclampsia Diagnostics Market Segments
By Product Type
- Instrument
- Consumables
By Test
- Blood Test
- Urine Test
By End-User
- Hospitals
- Specialty Clinics
- Diagnostic Centers
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































