RNA Therapeutics Market Size, Share & Trends Analysis Report By Therapy Type (mRNA Therapeutics, RNA interference (RNAi), Antisense oligonucleotides, Others), By Application (Oncology, Infectious Diseases, Genetic Diseases, Ophthalmology, Cardiovascular Diseases, Others), By End User (Hospitals & Clinics, Research Institutes, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
RNA Therapeutics Market Overview
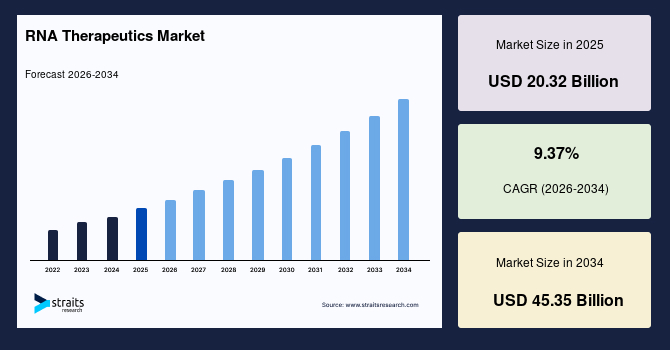
The global RNA therapeutics market size is valued at USD 20.32 billion in 2025 and is anticipated to grow from USD 22.15 billion in 2026 to USD 45.35 billion by 2034, growing at a CAGR of 9.37% from 2026-2034. The global RNA therapeutics market growth is attributed to the increasing incidences of cancers and rare genetic disorders, and the growing advancements in RNA delivery platforms that help to improve tissue targeting.
Key Market Trends & Insights
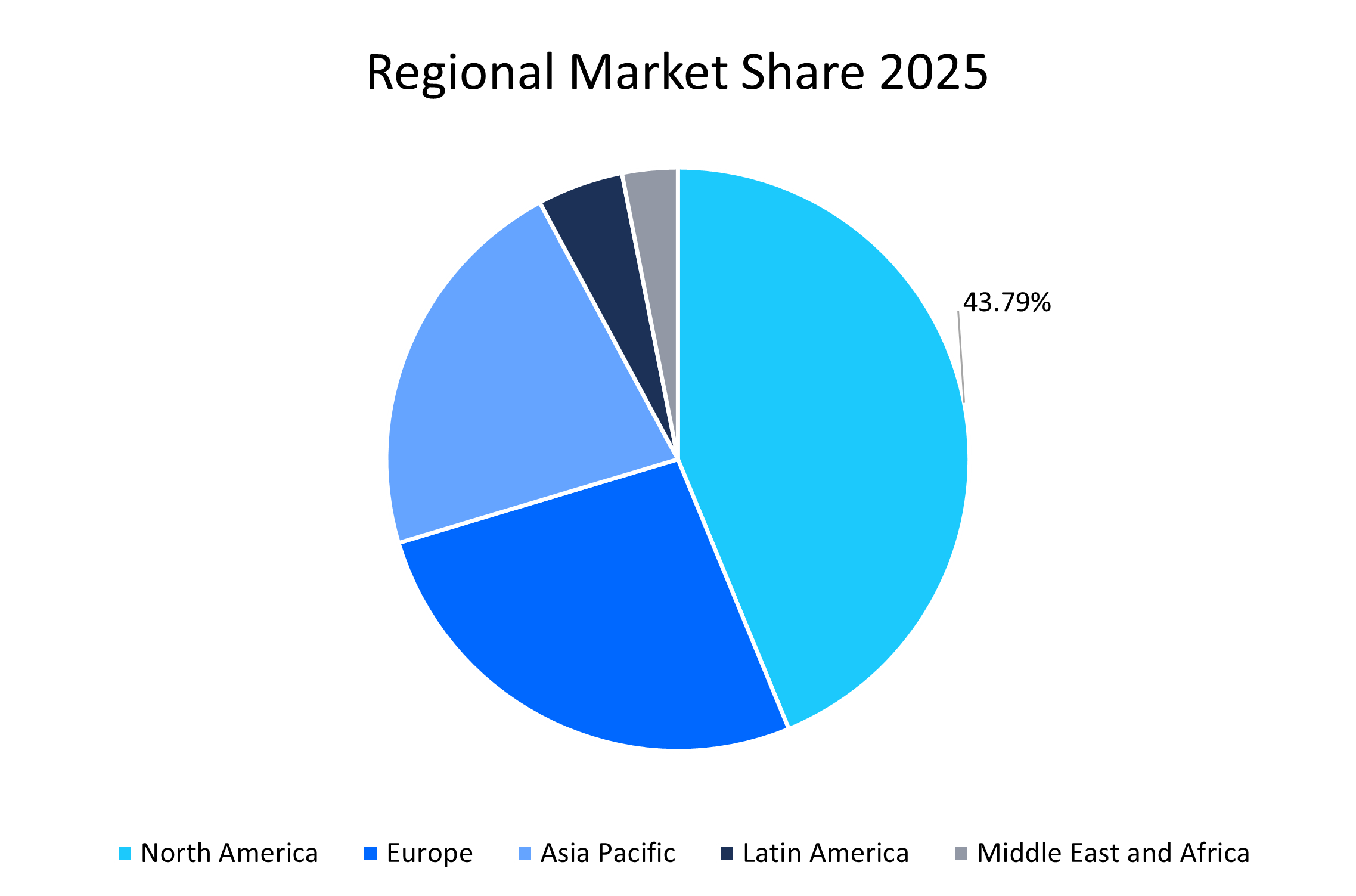
- North America held a dominant share of the global market, accounting for 43.79% share in 2025, due to new RNAi product approvals and a surge in incidence of new chronic diseases.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 11.64%, due to rising cancer prevalence, and countries focusing on the development of liver-targeting RNAi therapies.
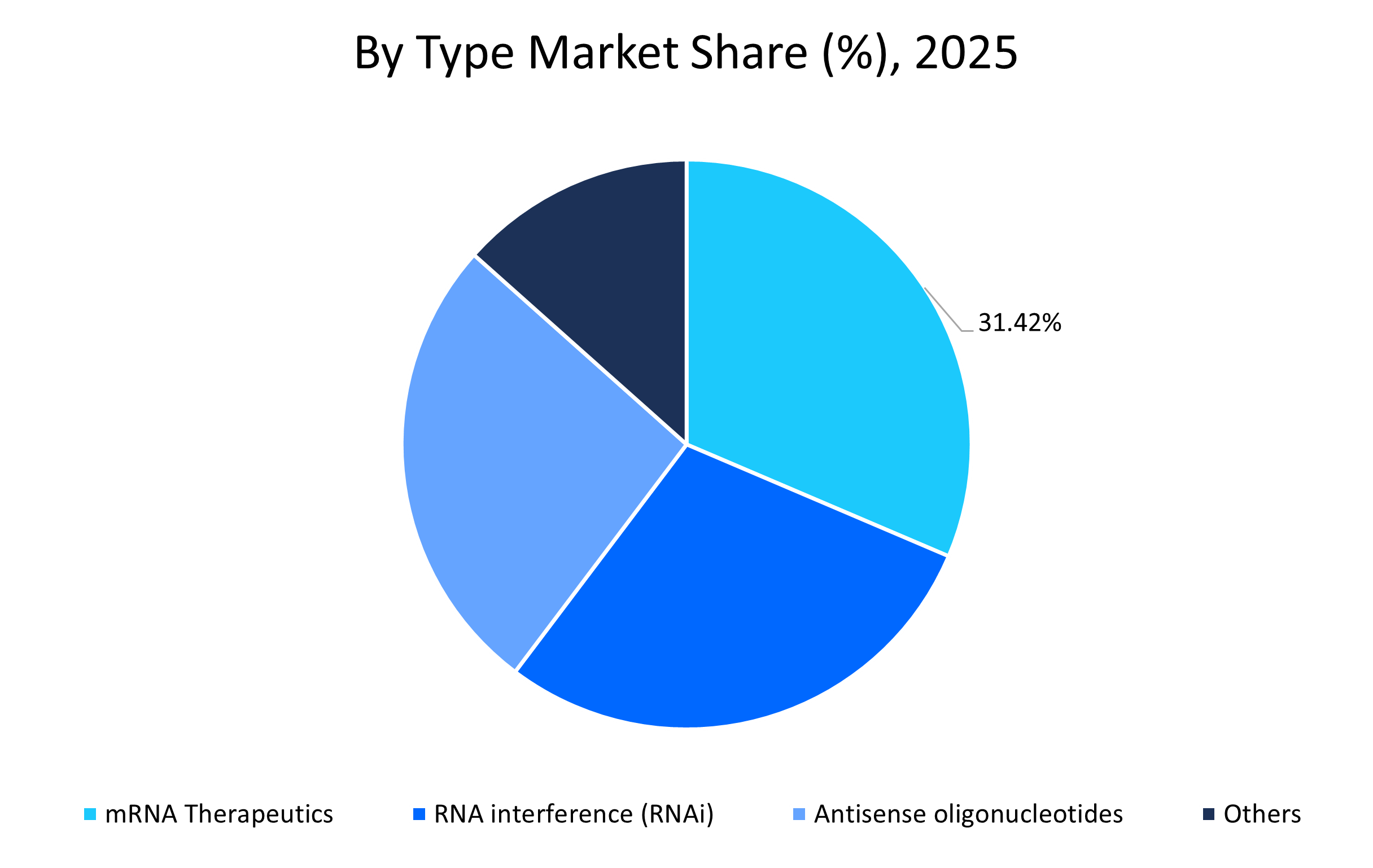
- Based on type, the mRNA therapeutics segment held the highest market share of 31.42% in 2025. This is attributed to the availability of approved vaccines in the market and a high percentage of drug candidates in clinical evaluation.
- Based on application, oncology is expected to register the fastest CAGR growth of 10.27%, owing to a surge in incidences of new cancer cases and adoption of RNA therapies for cancer management in developed countries.
- Based on end user, hospitals & clinics dominated the market in 2025.
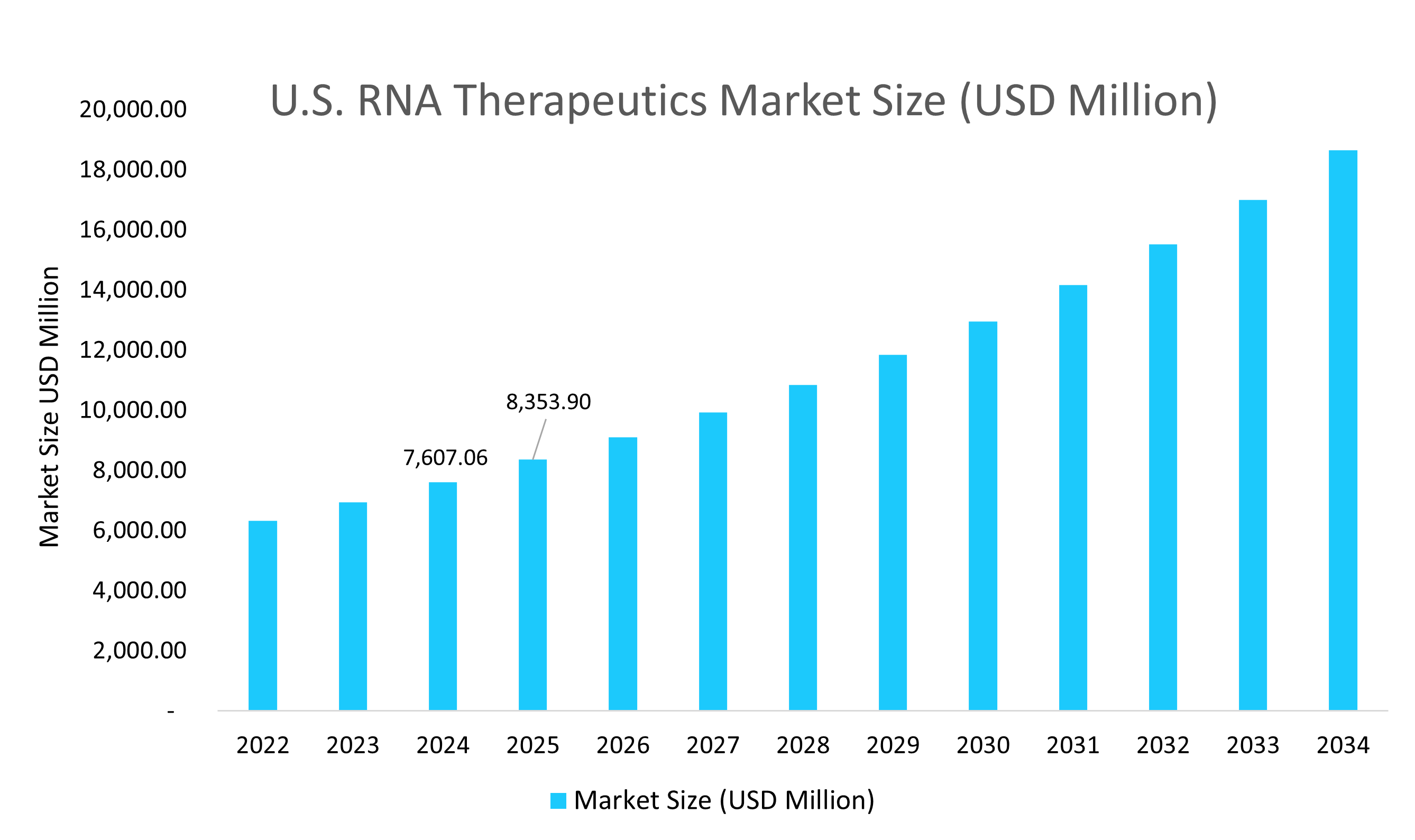
- The U.S. dominates the RNA therapeutics market, valued at USD 7.60 billion in 2024 and reaching USD 8.35 billion in 2025.
Market Size & Forecast
- 2025 Market Size: USD 20.32 billion
- 2034 Projected Market Size: USD 45.35 billion
- CAGR (2026-2034): 9.37%
- Dominating Region: North America
- Fastest-Growing Region: Asia-Pacific
Source: Straits Research Analysis
Approved RNA Therapies in 2023 & 2024
|
Product Name |
Manufacturer |
Approval Year |
|
COVID-19 vaccine |
CSPC Pharmaceutical |
2023 |
|
COVID-19 vaccine |
Sinocelltech |
2023 |
|
Qalsody |
Ionis Pharmaceuticals |
2023 |
|
Daichirona |
Daiichi Sankyo |
2023 |
|
Wainua |
Ionis Pharmaceuticals |
2023 |
|
Rivfloza |
Dicerna Pharmaceuticals |
2023 |
|
SYS-6006.32 |
CSPC Pharmaceutical |
2023 |
|
Rytelo |
Geron |
2024 |
|
mRESVIA |
Moderna Therapeutics |
2024 |
Source: American Society of Cell and Gene Therapy
Market Trends
Emergence of Circular Rna Therapies
The development of circular RNA and self-amplifying RNA platforms is a key trend for the market.
- For instance, in June 2024, Orna Therapeutics advanced its circRNA-based therapy ORN-101 into an early-stage trial for oncology.
- In parallel, Imperial College London’s saRNA vaccine work has shown robust immune responses at very low doses, underscoring dose efficiency and scalable manufacturing potential.
This showcases that next-generation RNA therapies are advancing, fueling investor interest and driving the growth of the RNA therapeutics market.
Strategic Collaborations between Key Market Players
Strategic collaboration is a key trend shaping the clinical innovations in RNA therapies.
- For instance, in May 2024, AbbVie Partnered with ADARx to co-develop next-generation siRNA therapies targeting neuroscience, immunology, and oncology indications. Under this deal, ADARx received a USD 335 million upfront payment for its RNA discovery platform.
Therefore, through this collaboration, AbbVie can accelerate the development of siRNA, which, in return, supports the market growth.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 20.32 Billion |
| Estimated 2026 Value | USD 22.15 Billion |
| Projected 2034 Value | USD 45.35 Billion |
| CAGR (2026-2034) | 9.37% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Moderna, Inc., Pfizer Inc., Alnylam Pharmaceuticals, Inc., Ionis Pharmaceuticals, Inc., Sarepta Therapeutics, Inc. |
to learn more about this report Download Free Sample Report
Market Drivers
Surge in Incidences of Cancer and Genetic Diseases
The rise in prevalence of cancer and genetic diseases is creating a strong demand for advanced therapies.
- For instance, according to the American Cancer Society, more than 2 million new cancer cases were reported in the U.S. in 2024, significantly increasing the need for novel RNA therapies.
Additionally, rare genetic diseases are rapidly surging; Alnylam’s Onpattro, the first approved RNA therapy, addresses these medical needs. These developments show that a growing patient pool is driving the product adoption.
Strong Funding Support from the Government and Academic Institutes
Increasing funding from government and academic institutes is a major factor driving the market growth.
- For instance, in April 2025, the U.S. National Institute of Health announced more than USD 200 million in new grants for advancing RNAi-based platforms for cancer and rare diseases.
These funding activities show that the government is making RNA innovation a strategic priority, which is expanding manufacturing capacity and accelerating market growth.
Market Restraint
High Development and Manufacturing Costs
The increasing cost of developing novel RNA therapies is restraining the market growth to some extent. Manufacturing mRNA and RNAi treatments requires high-purity nucleotides, specialized enzymes, and lipid nanoparticles.
- For instance, according to NCBI, about 60% of the cost of producing mRNA drugs comes from raw materials, making the manufacturing process costly and impacting the development of RNA therapies.
Market Opportunities
Expanding Pipeline of Mrna and Rnai Therapies
Leading vaccine developers have shifted their focus from only vaccines to developing RNA-based treatments for cancer, rare diseases, and other metabolic diseases.
Table: Alnylam RNA Therapeutics Pipeline
|
Product |
Indication |
Development Phase |
|
NUCRESIRAN |
ATTR Amyloidosis |
Phase 3 |
|
ZILEBESIRAN |
Hypertension |
Phase 3 |
|
MIVELSIRAN |
Cerebral Amyloid Angiopathy and Alzheimer’s Disease |
Phase 1/Phase 2 |
|
ALN-6400 |
Bleeding Disorders |
Phase 2 |
|
ELEBSIRAN |
Chronic HBV/HDV |
Phase 2/Phase 3 |
Source: Company Annual Reports & Straits Analysis
As more of these therapies move through clinical trials and gain approval, more treatment options will be available, making RNA therapeutics a major part of healthcare.
Regional Analysis
North America Market Trends
North America dominated the RNA therapeutics market in 2025, accounting for 43.79% market share. The growth is attributed to increased regulatory approvals and the development of novel RNA platforms. Furthermore, support from regulatory authorities also propels regional market growth. For instance, in 2024, the U.S. Food and Drug Administration (FDA) approved 21 RNA-based therapies, including mRNA vaccines and RNAi drug products. Moreover, the availability of robust infrastructure for product development and manufacturing also boosts market growth in the region.
Asia Pacific Market Growth Factors
Asis Pacific is emerging as the fastest-growing region with a CAGR of 12.7% from 2026-2034, owing to increasing local innovations, expanding contract development and manufacturing organizations, and a surge in infrastructure investments. For instance, in June 2024, CSPC Pharmaceutical Group in China secured approval for SYS6020 as the world’s first mRNA-LNP-based cell therapy. It is a synthetic mRNA therapy developed to treat melanoma. Additionally, Innorna received the IND clearance in July 2024 from NMPA and the U.S. FDA for its Herpes Zoster mRNA vaccine. Such advances are positioning the Asia Pacific as the fastest-growing region in the RNA therapeutics industry.
Source: Straits Analysis
Countries Analysis
U.s. Market Trends
U.S. accounts for the dominant share in the RNA therapeutics market, driven by increasing incidences of genetic and chronic diseases, robust R&D funding for developing novel RNA treatment approaches, advancements in synthetic biology and bioinformatics, and internal partnerships for product development.
- For instance, in August 2024, Sirnaomics entered a partnership with Gore Range for the establishment of a joint venture, Sagesse Bion, Inc., to advance its novel RNAi therapeutic products into aesthetic medicine.
Moreover, the FDA’s leadership in approving new RNA therapies bolsters manufacturers’ confidence to advance their pipelines.
China Market Growth Factors
China RNA therapeutics market growth is driven by a rapid increase in clinical trials of mRNA and RNAi drugs, led by domestic manufacturers such as Stemirna Therapeutics and Suzhou Ribo Life Science. For instance, in 2023, Stemirna Therapeutics advanced its mRNA COVID-19 booster into the last stage of clinical trials. This showcases China’s ability to rapidly develop and scale mRNA vaccines. Moreover, the Chinese government has made biotechnology a priority in its five-year plan, which will enable rapid funding, infrastructure support, and regulatory approvals for RNA therapeutics.
Germany Market Trends
Germany's market for RNA therapeutics growth is attributed to robust biotech firms and active support of the government sector through streamlining clinical research, offering funding for constructing advanced R&D facilities, and research. For instance, in June 2025, Neumirna Therapeutics secured USD 23 million in series A funding for the development of RNA therapies to treat epilepsy and other neurological diseases.
Japan Market Growth Factors
In Japan, the RNA therapeutics industry is gaining momentum through joint ventures for developing RNA therapies, a focus on mRNA technologies, and strong government support for biotech industry development.
- For instance, in March 2024, Tokyo Medical and Dental University researchers developed chemically modified mRNA technologies for cancer vaccine development.
- Additionally, in April 2025, Ono Pharmaceuticals entered a partnership with U.S. startup Jorna Therapeutics to speed up the development of RNA therapies on Ono’s scale and Jorna’s technology.
Uk Market Trends
Growth of the RNA therapeutics market in UK is supported by a leading genomic research ecosystem, strong academic and industry collaborations. Additionally, the UK’s NHS framework supports the integration of advanced therapies into public healthcare with pilot programs testing mRNA vaccines for cancer patients directly in the NHS hospitals. Thus, all aforementioned factors create a favorable environment for the growth of the RNA therapeutics market.
Market Segmentation
Type Insights
The mRNA therapeutics dominated the market with a revenue share of 31.42% in 2025. This growth is attributed to the strong success rate of mRNA vaccines and the rapid expansion of these therapies in oncology and cardiovascular disease management. Moreover, robust investments across the research and development of mRNA therapies make it a dominant segment in the market.
Source: Straits Analysis
Application Insights
The oncology segment dominated the market in 2025 with a revenue share of 33.57% and is anticipated to register the fastest CAGR growth. The growth is attributed to rising global cancer burden, the strong potential of RNA-based targeted therapies, and continued advances in mRNA cancer vaccines, all of which are accelerating clinical uptake and pipeline investment.
End User Insights
The hospitals and clinics segment dominated the market in 2025, as these are the primary healthcare facilities for administering advanced mRNA vaccines and RNAi treatments. Additionally, the majority of the clinical trials and early access programs for RNA therapies are conducted in hospital settings, allowing faster access to novel drugs. These factors support segmental growth.
Company Market Share
The global RNA therapeutics market is moderately consolidated, with fewer companies dominating the revenue share. However, there is an emergence of new biotech innovators having RNA therapeutics under clinical evaluation. The top players in the industry are Moderna Inc., Alnylam Pharmaceuticals, Ionis Pharmaceuticals, and Pfizer Inc.
The industry participants are inclined towards various market strategies such as product approval, collaboration, funding, merger, and acquisition to remain competitive in the market.
City Therapeutics Inc.: An emerging market player
City Therapeutics is an emerging developer of RNA interference-based medicines. The company is actively engaged in the development of the next generation of small interfering RNA. The company is emerging as a significant player in the market through a funding grant.
- In October 2024, City Therapeutics received USD 135 million Series A funding from ARCH Venture Partners to develop novel RNAi-based medicines.
List of Key and Emerging Players in RNA Therapeutics Market
- Moderna, Inc.
- Pfizer Inc.
- Alnylam Pharmaceuticals, Inc.
- Ionis Pharmaceuticals, Inc.
- Sarepta Therapeutics, Inc.
- Novartis AG
- Sanofi
- CureVac N.V.
- Orna Therapeutics
- Arrowhead Pharmaceuticals, Inc.
- Suzhou Abogen Biosciences
- CSPC Pharmaceutical
- Dicerna Pharmaceuticals
- Geron
- Others
to learn more about this report Download Market Share
Recent Development
- August 2025: Pfizer Inc. received the supplemental Biologics License Application (sBLA) for COMIRNATY, an mRNA vaccine for COVID-19 in adults aged 65 and older.
- May 2025: Biogen Inc. and City Therapeutics, Inc. have collaborated to develop novel RNAi therapies. Under this collaboration, both companies aim to develop a target to treat CNS system diseases.
- In March 2025, the FDA approved Alnylam’s AMVUTTRA® (vutrisiran) for the treatment of transthyretin amyloid cardiomyopathy (ATTR-CM), marking the first RNA interference (RNAi) therapeutic authorized for this cardiac indication.
- In May 2025, the FDA approved Moderna’s mNEXSPIKE (mRNA-1283) vaccine for older adults and certain high-risk groups, marking a significant milestone for next-generation mRNA therapeutics and sustaining commercial demand.
- In June 2025, BioNTech acquired CureVac to strengthen its mRNA-based oncology and therapeutic vaccine programs, reflecting continued investment and consolidation in the RNA therapeutics market.
- In August 2025, the FDA approved Ionis’ donidalorsen (Dawnzera), an antisense oligonucleotide, for the prevention of hereditary angioedema, marking a significant expansion of RNA-targeted therapies in rare disease treatment.
Analyst Opinion
As per the analyst's opinion RNA therapeutics market is rapidly expanding and has high growth potential due to the dominance of mRNA therapies, the emergence of new RNAi treatments, and the shift towards diversification.
- For instance, new biotech innovators are focusing on wide disease areas such as cardiovascular, autoimmune diseases, and CNS disorders. This shift towards diversification is a major factor supporting the adoption of RNA therapies in expanded disease indications. Thus, the RNA therapeutic market is driven by the emergence of new therapies and indication expansion.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 20.32 Billion |
| Market Size in 2026 | USD 22.15 Billion |
| Market Size in 2034 | USD 45.35 Billion |
| CAGR | 9.37% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Therapy Type, By Application, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
RNA Therapeutics Market Segments
By Therapy Type
- mRNA Therapeutics
- RNA interference (RNAi)
- Antisense oligonucleotides
- Others
By Application
- Oncology
- Infectious Diseases
- Genetic Diseases
- Ophthalmology
- Cardiovascular Diseases
- Others
By End User
- Hospitals & Clinics
- Research Institutes
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Dhanashri Bhapakar
Senior Research Associate
Dhanashri Bhapakar is a Senior Research Associate with 3+ years of experience in the Biotechnology sector. She focuses on tracking innovation trends, R&D breakthroughs, and market opportunities within biopharmaceuticals and life sciences. Dhanashri’s deep industry knowledge enables her to provide precise, data-backed insights that help companies innovate and compete effectively in global biotech markets.










































