Stem Cell Assays Market Size, Share & Trends Analysis Report By Assay Type (Cell Viability Assay, Isolation & Purification Assay, Cell Identification Assay, Proliferation Assay, Differentiation Assay, Apoptosis Assay, Others), By Product & Service (Instruments, Kits & Reagents, Services), By Cell Type (Adult stem cells, Hematopoietic Stem Cell, Embryonic stem cells, Induced pluripotent stem cells, Neural Stem Cell, Others), By Application (Regenerative Medicine & Therapy Development, Drug Discovery, Clinical Research, Others), By End Use (Pharmaceutical and Biotechnology Companies, Contract Research Organizations, Academic and Research Institutions) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Stem Cell Assays Market Overview
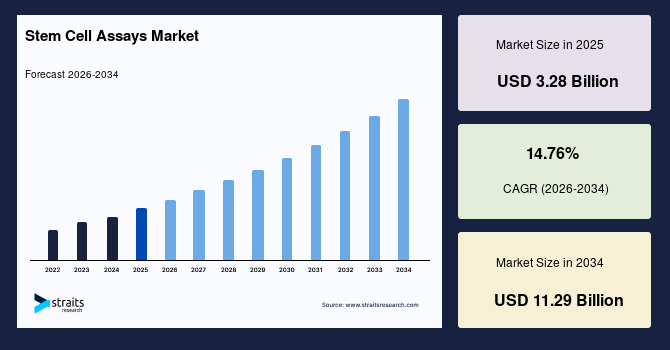
The global stem cell assays market size is valued at USD 3.28 billion in 2025 and is estimated to reach USD 11.29 billion by 2034, growing at a CAGR of 14.76% during 2026-2034. The global market observed impressive growth, driven by the expanding use of stem cell-derived organoids as predictive micromodels for rare disease drug testing.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 37.41% in 2025.
- The Asia Pacific region is estimated to grow at the fastest pace, with a CAGR of 16.38% from 2026-2034.
- Based on assay type, the differentiation assay segment is expected to register the fastest CAGR of 15.54% during 2026-2034.
- Based on product & service, the kits & reagents segment dominated the market, accounting for 48.76% revenue share in 2025.
- By cell type, adult stem cells segment dominated the market with a revenue share of 46.08% in 2025.
- Based on the application, the Adult stem cells segment dominated the market with a revenue share of 46.08% in 2025.
- Based on end use, the pharmaceutical and biotechnology companies segment dominated the market in 2025.
- The U.S. dominates the market, valued at USD 960.29 million in 2024 and reaching USD 1,097.90 million in 2025.
Table: U.S. Stem Cell Assays Market Size (USD Million)
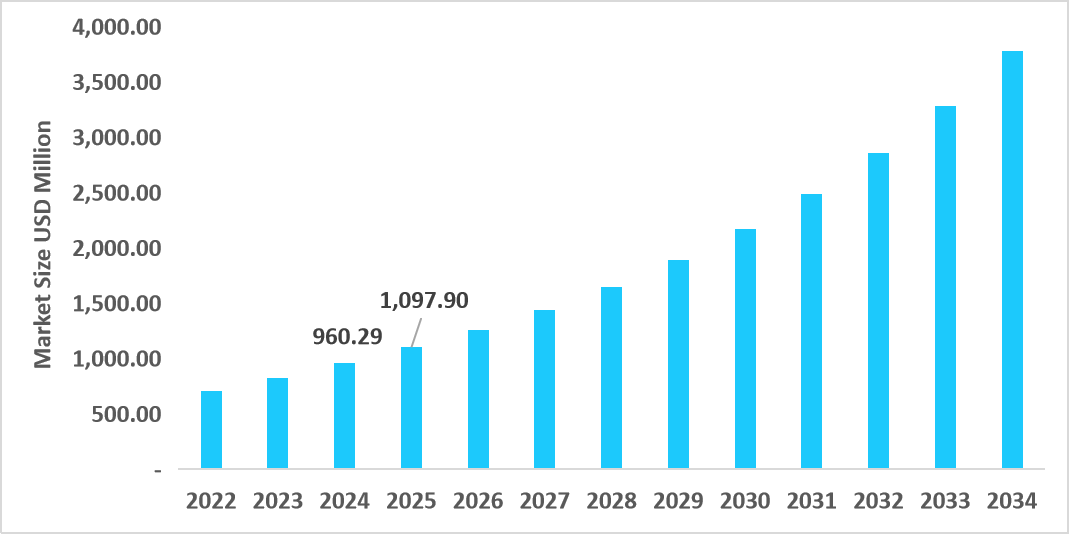
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 3.28 billion
- 2034 Projected Market Size: USD 11.29 billion
- CAGR (2026-2034): 14.76%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The stem cell assays market encompasses a wide range of analytical tools and technologies used to evaluate stem cell function. It includes diverse assay types such as viability, proliferation, differentiation, apoptosis, isolation, and identification assays, supported by instruments, specialized kits, reagents, and services. These solutions are applied to various cell categories, including adult, embryonic, hematopoietic, neural, and iPSC lines, and are widely utilized in regenerative medicine, drug discovery, and clinical research by pharmaceutical firms, CROs, and academic institutions.
Latest Market Trends
Rising Adoption of iPSC-Derived Disease Models
A key trend in the stem cell assays market is the rapid adoption of induced pluripotent stem cell derivated disease models, driven by increasing demand for physiologically relevant testing systems in drug discovery. Recent advancements in reprogramming efficiency and genome editing tools such as CRISPR are enabling the creation of highly specific patient derived cell lines, enhancing assay precision. This shift is accelerating personalized medicine research and strengthening the market’s technological evolution.
Surge in Automated High Throughput Stem Cell Assays
The increasing adoption of automated high throughput screening platforms tailored for stem cell workflows is a major trend for market growth. This shift reflects demand for faster, more reproducible screening across large compound libraries and complex 3D or iPSC derived models, enabling biopharma and CROs to accelerate drug discovery and reduce manual variability in testing protocols.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 3.28 Billion |
| Estimated 2026 Value | USD 3.75 Billion |
| Projected 2034 Value | USD 11.29 Billion |
| CAGR (2026-2034) | 14.76% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Thermo Fisher Scientific Inc., Merck KGaA, Danaher, BD, Bio-Rad Laboratories, Inc. |
to learn more about this report Download Free Sample Report
Market Drivers
Growing Integration of Stem Cell Assays in Drug Toxicity Testing
A key driver of the stem cell assays market is the rising use of stem cell-based models for early toxicity screening as pharmaceutical companies prioritize safer and faster drug development. With increasing regulatory pressure to reduce late-stage trial failures, laboratories are adopting human-based iPSC cardiotoxicity and hepatotoxicity assays to predict adverse effects earlier in the pipeline. This shift toward predictive safety testing is notably boosting demand across biopharma R&D programs.
Market Restraints
High Cost and Technical Complexity of Advanced Stem Cell Assay Systems
A restraint in the global market is the rising cost and technical sophistication of assay platforms. Many advanced systems, mainly those using 3D cultures or high-throughput automation, require expensive reagents, skilled personnel, and specialized infrastructure. Recent industry assessments indicate that assay setup expenses have increased by more than 20% since 2022, limiting adoption among smaller laboratories and creating financial barriers that slow overall market expansion.
Market Opportunity
Growing Commercialization of iPSC Assay Platforms in Precision Medicine
A key opportunity in the stem cell assays market is the rapid commercialization of induced pluripotent stem cell-based assay platforms driven by rising demand for patient-specific disease modeling. With precision medicine investments increasing globally, supported by multiple biopharma companies expanding iPSC partnerships since 2023, developers are accelerating the launch of ready-to-use iPSC assay kits and high-content testing solutions. This shift is opening new revenue channels and broadening applications across drug discovery and advanced therapeutic research.
Regional Analysis
North America dominated the stem cell assays market in 2025, accounting for 37.41% market share. This growth is driven by the prevalence of world leading research hubs and scientific institutions such as the WiCell Research Institute, which supplies globally used human pluripotent stem cell lines and protocols, boosting regional assay demand and innovation. These centers, combined with dense biotech clusters and high research output, uniquely strengthen North American market leadership.
In Canada the market growth is supported by expansion of STEMCELL Technologies, a Vancouver based global biotech leader in cell culture media, separation technologies, and assay tools, which recently strengthened its presence in Toronto’s innovation hub at the MaRS Discovery District, enhancing regional research capacity and commercialization of advanced stem cell solutions.
Asia Pacific Market Insights
Asia Pacific is emerging as the fastest-growing region with a CAGR of 16.38% from 2026-2034. The growth is driven by the rapid expansion of regional clinical research hubs and partnerships, especially China’s substantial stem cell R&D investment, which has established advanced assay facilities and accelerated therapeutic validation studies. These government-backed innovation centers are increasing assay utilization and attracting multinational biotech collaborations across the region.
The Japanese stem cell assays market is expanding due to patient-specific iPSC production facilities, such as Kyoto University’s automated “iPS cell factory,” which lowers production cost and accelerates the supply of clinical‑grade cells for research and trials. This regional scientific infrastructure enhances assay utilization in drug development and regenerative medicine.
Regional Market share (%) in 2025
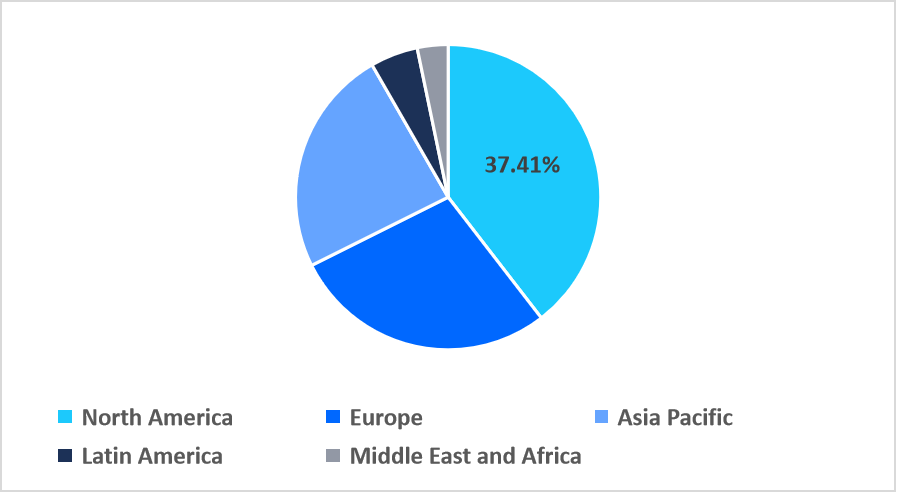
Source: Straits Research
Europe Market Insights
In Europe, the market growth is accelerated by the European Bank for Induced Pluripotent Stem Cells (EBiSC), which supplies over 800 quality-controlled iPSC lines for disease research. This standardized regional repository enhances collaboration across EU research labs and biotechs, boosting assay utilization for drug discovery and regenerative studies.
In Germany, the stem cell assays market is experiencing growth due to the presence of specialized stem cell process engineering centers, such as the Fraunhofer Project Center for Stem Cell Process Engineering, which integrates automation, innovative materials, and bioreactor technologies to scale stem cell production and analytical workflows. This research to industry infrastructure enhances assay development, attracts biotech partnerships, and strengthens Germany’s advanced regenerative research ecosystem.
Latin America Market Insights
In Latin America, the stem cell assays market growth is propelled by the emergence of regional manufacturing hubs in Brazil that lower import dependency and enhance affordability through localized production. Additionally, the integration of regenerative medicine approaches, combining implants with stem cell-enriched fat grafting, is gaining clinical traction and expanding procedural acceptance across the region.
In Brazil, the market growth is supported by Brazil’s strong academic industry collaboration, particularly between São Paulo’s major universities and regional biotech firms, which accelerates translational stem cell research and commercial assay adoption. This localized research ecosystem enhances assay utilization for drug discovery and regenerative studies across the region.
Middle East and Africa Market Insights
In the Middle East, the market is expanding due to rapid establishment of advanced stem cell research and manufacturing hubs such as the Dubai Healthcare City (DHCC) GMP facility, which supports local production and cryogenic banking of stem cells. This high‑grade infrastructure attracts regional and international research activity, boosting demand for specialized assays.
In Saudi Arabia, the stem cell assays market is growing due to the launch of the King Faisal Specialist Hospital & Research Centre’s first gene and cell therapy manufacturing facility, enabling local production of advanced stem‑cell and CAR‑T therapies under GMP standards. This landmark infrastructure strengthens domestic R&D, attracts scientific talent, and accelerates clinical validation efforts within the Kingdom’s biotechnology ecosystem.
Assay Type Insights
The cell viability assay segment dominated the market in 2025 with a revenue share of 32.69%. This growth is driven by the rising use of miniaturized microfluidic viability panels that enable single-cell metabolic monitoring. These platforms allow researchers to assess heterogeneous cell responses with exceptional precision, increasing demand for advanced viability-focused analytical tools.
The differentiation assay segment is projected to witness the fastest CAGR of 15.54% during the forecast timeframe. This growth is augmented by the growing adoption of lineage-specific fluorescent barcoding systems that track transition states during stem cell maturation. This innovation enables precise mapping of micro-differentiation pathways, increasing demand for specialized differentiation-focused assay solutions.
By Assay Type Market Share (%), 2025
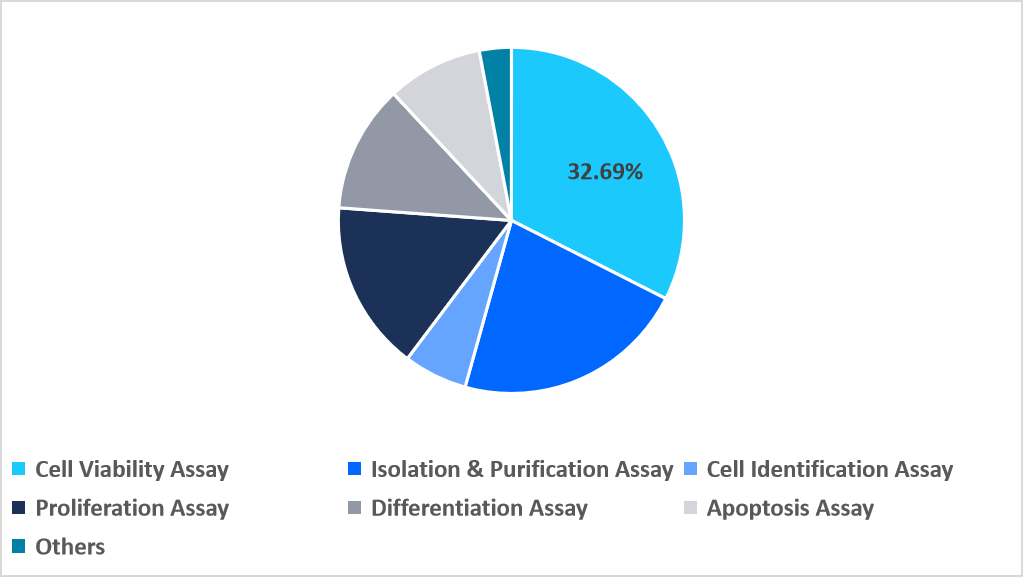
Source: Straits Research
Product & Service Insights
The kits & reagents segment dominated the market in 2025 with a revenue share of 48.76%, owing to the increasing demand for pre-validated stem cells specific enzyme blends that ensure uniform dissociation of fragile iPSC colonies. These precision-engineered formulations reduce variability, boosting reliance on specialized kits and reagent systems.
The instruments segment is expected to register the fastest CAGR growth of 15.32% during the forecast period. This growth is supported by growing adoption of instruments, such as Agilent xCELLigence Cell Analysis (RTCA) system, which is gaining rapid adoption because it measures cell proliferation, adhesion, and differentiation continuously without labels.
Cell Type Insights
The adult stem cells segment dominated the market in 2025 with a revenue share of 46.08%, due to increasing use of mesenchymal stem cells for regenerative therapies and personalized medicine. Their ease of isolation from bone marrow, adipose tissue, and umbilical cord, combined with low ethical concerns, has accelerated adoption in drug testing and clinical research.
Application Insights
The drug discovery segment dominated the market in 2025, with a revenue share of 44.13%. This growth is driven by CRISPR-engineered stem cell lines for target validation, allowing researchers to rapidly model genetic diseases and test compound effects, providing highly specific and predictive insights for early-stage drug development.
End Use Insights
The pharmaceutical and biotechnology companies segment dominated the market in 2025 because of the increasing adoption of CRISPR-Cas9 gene edited stem cell lines. These technologies allow precise disease modeling and targeted drug screening, enabling companies to develop safer and more effective therapies, driving large-scale adoption of stem cell assays.
Competitive Landscape
The global stem cell assays market is moderately consolidated, with few key players commanding major revenue through continuous innovation in assay platforms and automation technologies. Leading companies, including Thermo Fisher Scientific, Danaher, Merck KGaA, Bio‑Techne, and STEMCELL Technologies, are investing in partnerships, product launches, and expanded service offerings. These strategic initiatives enhance competitive differentiation and support broader adoption across pharmaceutical, biotech, and academic research sectors.
Bit Bio Ltd.: An emerging market player
Bit Bio Ltd. is an emerging player in the market, specializing in synthetic biology–driven production of human iPSC-derived cell types. The company offers a proprietary opti-ox platform that generates consistent, high-quality cells for research and drug discovery applications.
- For instance, in March 2025, Bit Bio partnered with a leading European biopharma firm to supply standardized iPSC-derived neuronal cells for drug screening studies.
List of Key and Emerging Players in Stem Cell Assays Market
- Thermo Fisher Scientific Inc.
- Merck KGaA
- Danaher
- BD
- Bio-Rad Laboratories, Inc.
- PerkinElmer, Inc.
- Bio-Techne Corporation
- STEMCELL Technologies Inc.
- Lonza Group Ltd.
- Corning Incorporated
- Promega Corporation
- Agilent Technologies, Inc.
- Miltenyi Biotec
- Cytiva
- Sartorius AG
- Abcam plc
- Cell Signaling Technology, Inc.
- Takara Bio Inc.
- Charles River Laboratories International, Inc.
- Others.
Strategic Initiatives
- October 2025: REPROCELL Europe Ltd. launched the Alvetex plasticware platform designed to expand the capabilities of 3D cell culture and bioengineered tissue models.
- January 2024: Cellcolabs AB. and REPROCELL Inc. partnered to globally distribute the high-quality MSCs and MSC derivatives manufactured by Cellcolabs for research and clinical applications.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 3.28 Billion |
| Market Size in 2026 | USD 3.75 Billion |
| Market Size in 2034 | USD 11.29 Billion |
| CAGR | 14.76% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Assay Type, By Product & Service, By Cell Type, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Stem Cell Assays Market Segments
By Assay Type
- Cell Viability Assay
- Isolation & Purification Assay
- Cell Identification Assay
- Proliferation Assay
- Differentiation Assay
- Apoptosis Assay
- Others
By Product & Service
- Instruments
- Kits & Reagents
- Services
By Cell Type
- Adult stem cells
- Hematopoietic Stem Cell
- Embryonic stem cells
- Induced pluripotent stem cells
- Neural Stem Cell
- Others
By Application
- Regenerative Medicine & Therapy Development
- Drug Discovery
- Clinical Research
- Others
By End Use
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations
- Academic and Research Institutions
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































