Stem Cell Culture Media Market Size, Share & Trends Analysis Report By Cell Line (Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs), Induced Pluripotent Stem Cells (iPSCs), Embryonic Stem Cells (ESCs), Other Stem Cells), By Application (Drug Discovery & Development, Stem Cell Therapy Manufacturing, Tissue Engineering & Regenerative Medicine), By End-use (Pharmaceutical & Biotechnology Companies, CDMOs & CROs, Stem Cell Banks / Biobanks, Hospitals & Transplant Centers, Academic & Research Institutes) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Stem Cell Culture Media Market Overview
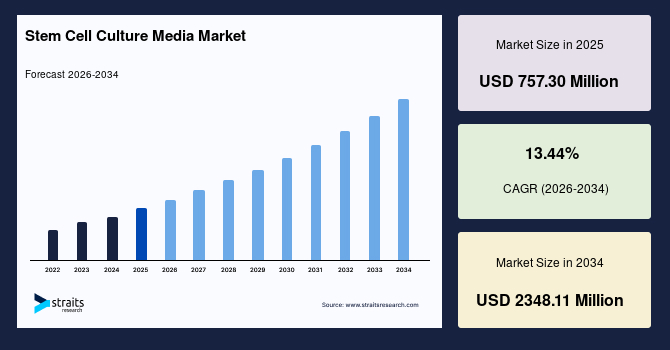
The global stem cell culture media market size is valued at USD 757.30 million in 2025. It is estimated to reach USD 2348.11 million by 2034, growing at a CAGR of 13.44% during the forecast period. The consistent market growth is supported by expanding clinical translation of stem cell therapies alongside rising adoption of defined and serum-free culture media in regenerative medicine research.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 39.14% in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 15.44%.
- Based on Cell Line, mesenchymal stem cells (MSCs) dominated the market with a revenue share of 49.23%.
- Based on the Application, drug discovery & development dominated the market with 50.12%.
- Based on End-Use, the pharmaceutical & biotechnology companies segment dominated the market with a revenue share of 39.33%.
- The U.S. dominates the stem cell culture media market, valued at USD 236.97 million in 2024 and reaching USD 267.80 million in 2025.
Table: U.S. Stem Cell Culture Media Market Size (USD Million)
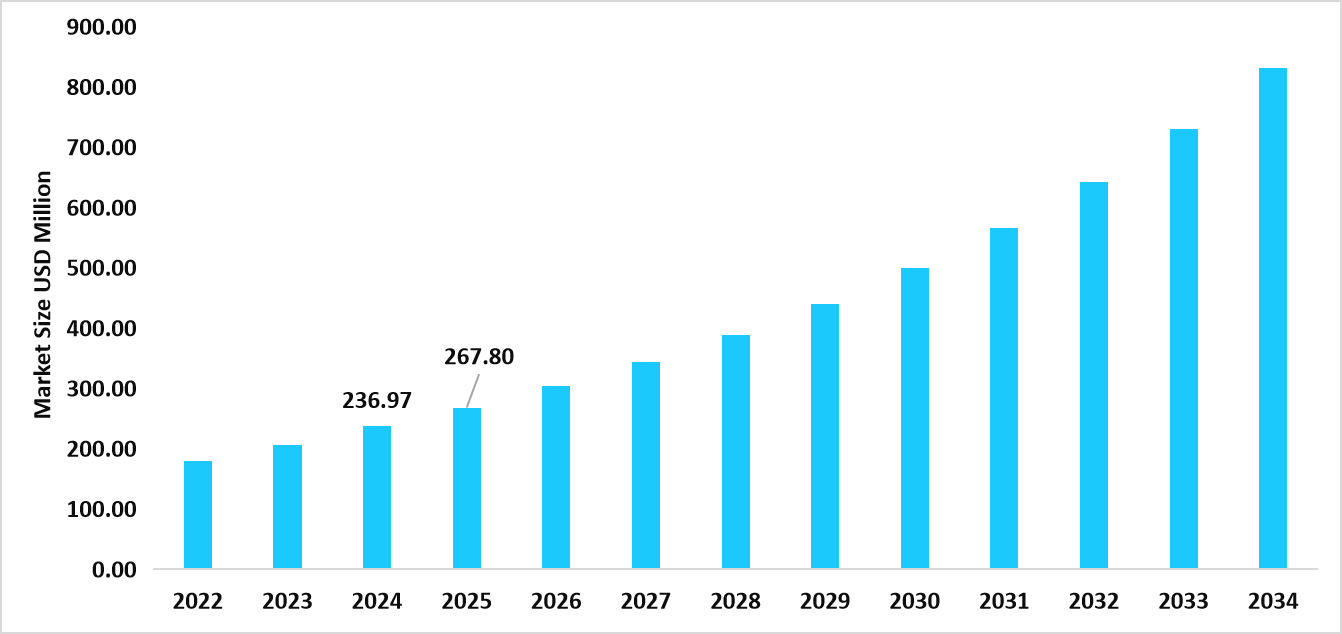
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 757.30 million
- 2034 Projected Market Size: USD 2348.11 million
- CAGR (2026-2034): 13.44%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The stem cell culture media market comprises specialized nutrient formulations used to support the growth, maintenance, expansion, and controlled differentiation of stem cells across research, translational, and clinical manufacturing settings. These media are formulated to meet the specific biological requirements of different stem cell types, including mesenchymal stem cells, hematopoietic stem cells, induced pluripotent stem cells, embryonic stem cells, and other stem cell lines, enabling consistent cell performance and scalability. By application, stem cell culture media are widely used in drug discovery and development, stem cell therapy manufacturing, and tissue engineering and regenerative medicine, where precise media composition is required to ensure reproducibility and regulatory alignment. By end use, the market caters to pharmaceutical and biotechnology companies, CDMOs and CROs, stem cell banks and biobanks, hospitals and transplant centers, and academic and research institutes, reflecting the expanding adoption of stem cell technologies across both research and therapeutic value chains.
Market Trends
Shift From Serum-Based Culture Media to Chemically Defined and Xeno-free Formulations
Stem cell culture practices are increasingly transitioning from serum-containing media toward chemically defined and xeno-free formulations to achieve tighter control over cellular microenvironments. Serum-based systems introduce variability linked to donor source and processing, which can influence proliferation rates, differentiation efficiency, and genetic stability. Defined formulations allow precise regulation of growth factors, cytokines, and metabolic substrates, supporting consistent expansion of mesenchymal stem cells, hematopoietic stem cells, induced pluripotent stem cells, and embryonic stem cells. This shift also aligns culture workflows with clinical manufacturing expectations, where traceability and composition transparency are central to process standardization.
Shift From General-purpose Media to Cell Line and Application-specific Media Platforms
The market is also moving from broadly applicable stem cell media toward formulations engineered for specific cell lines and downstream uses. Media designed for drug discovery emphasize rapid expansion and phenotypic consistency, while formulations for stem cell therapy manufacturing prioritize lineage stability and long-term culture performance. In tissue engineering and regenerative medicine, application-specific media support matrix interaction and controlled differentiation pathways. This shift reflects increasing specialization across stem cell workflows, where tailored media improve alignment between culture conditions and functional outcomes.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 757.30 Million |
| Estimated 2026 Value | USD 856.35 Million |
| Projected 2034 Value | USD 2348.11 Million |
| CAGR (2026-2034) | 13.44% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Thermo Fisher Scientific Inc., Merck KGaA, Cytiva, Lonza Group AG, Takara Bio Inc. |
to learn more about this report Download Free Sample Report
Market Drivers
Acceleration of Translational and Commercial Stem Cell Programs
Advancement of stem cell therapies from research into clinical development and commercial manufacturing is driving demand for standardized culture media. Therapy developers require scalable media systems that maintain consistent cell characteristics across multiple production batches. Pharmaceutical and biotechnology companies, along with CDMOs, increasingly invest in media solutions that integrate smoothly into controlled manufacturing environments and support regulatory review processes.
Market Restraint
Process Lock-in and Compatibility Challenges in Regulated Settings
The stem cell manufacturing workflows are established, and altering culture media involves extensive comparability assessments to confirm unchanged cell identity, safety, and functional attributes. These studies require time, resources, and regulatory documentation, creating hesitation toward adopting alternative media even when performance advantages are recognized. This constraint slows market penetration of newer formulations, which further restrains the market growth.
Market Opportunities
Growth Of Distributed Stem Cell Production and Storage Networks
Expansion of stem cell banks, biobanks, and regional production facilities creates opportunities for culture media suppliers to deliver standardized and scalable solutions. These centers manage diverse stem cell lines for clinical, research, and therapeutic use, favoring media that support uniform expansion and storage compatibility. As decentralized manufacturing and storage models expand, demand for adaptable stem cell culture media is positioned to rise.
Regional Analysis
North America represents a leading region in the stem cell culture media market with a share of 39.14%, supported by extensive integration of stem cell research within academic institutes, biopharmaceutical companies, and clinical development programs. The region demonstrates strong utilization of standardized culture media across early-stage research and translational manufacturing workflows. Widespread availability of advanced laboratory infrastructure and established cell therapy pipelines supports continuous consumption of specialized media. Collaboration between research institutions, biotechnology firms, and manufacturing partners strengthens regional market positioning.
In the U.S., market expansion is supported by growing incorporation of stem cell platforms into drug screening programs and cell-based therapeutic development. Research centers and biopharmaceutical manufacturers adopt scalable media systems to support consistent expansion and differentiation, reinforcing the country’s leadership.
Asia Pacific Market Insights
Asia Pacific registers the fastest growth at 15.44%, driven by the rapid expansion of stem cell research facilities and the rising establishment of regional cell therapy manufacturing centers. Laboratories across urban and semi-urban areas increase adoption of stem cell culture workflows to support translational research and clinical studies. The growth of domestic life science suppliers and regional research networks accelerates market development.
In China, expansion is supported by large-scale investment in stem cell research institutes and clinical study programs. Increased use of standardized culture media in hospital-affiliated research centers and manufacturing facilities contributes to sustained growth.
Regional Market share (%) in 2025
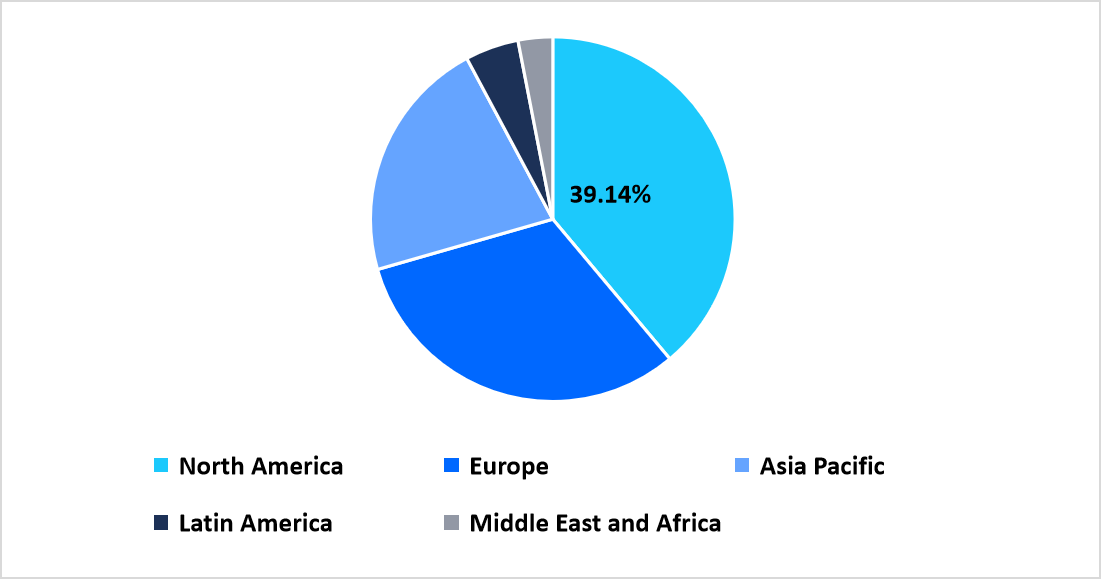
Source: Straits Research
Europe Market Insights
Europe maintains steady adoption through the structured integration of stem cell research within national healthcare systems and academic networks. Research laboratories and biopharmaceutical organizations utilize stem cell culture media to support disease modeling and regenerative medicine programs. Emphasis on harmonized research standards across countries supports consistent uptake.
In Germany, growth is driven by the increasing application of stem cell models in translational research and pharmaceutical development. Adoption of standardized culture media within research institutes and manufacturing facilities supports market stability.
Latin America Market Insights
Latin America experiences a gradual expansion as research institutions and healthcare organizations broaden engagement with stem cell research activities. Universities and public laboratories increasingly incorporate stem cell culture workflows into biomedical research programs. Adoption of standardized media supports consistent laboratory practices across the region.
In Brazil, growth is reinforced by the rising use of stem cell research platforms within academic centers and hospital-affiliated laboratories. Expansion of research capacity supports broader regional participation.
Middle East and Africa Market Insights
The Middle East and Africa region advances through the strengthening of biomedical research infrastructure and increasing focus on regenerative medicine programs. Research institutes and hospitals expand the adoption of stem cell culture systems to support translational studies. Capacity development initiatives and academic collaborations contribute to wider regional uptake.
In the United Arab Emirates, market momentum is supported by the integration of stem cell research platforms within research institutes and specialized healthcare centers. Expansion of laboratory capabilities strengthens the country’s regional presence.
Cell Line Insights
Mesenchymal stem cells dominated the cell line segment with a share of 49.23%, supported by their broad use across regenerative medicine research, immunomodulatory studies, and allogeneic therapy development. Widespread adoption of mesenchymal stem cells in scalable expansion protocols and their compatibility with standardized culture systems sustain higher consumption of dedicated media formulations.
Induced pluripotent stem cells are anticipated to register the fastest growth at 14.56%, driven by increasing use in disease modeling, toxicity screening, and personalized medicine workflows. Rising interest in reprogramming technologies and long-term pluripotency maintenance accelerates demand for specialized media designed for induced pluripotent stem cell culture.
Application Insights
Drug discovery and development dominated the application segment with a share of 50.12%, reflecting extensive use of stem cell-derived models in target validation, compound screening, and safety profiling. Integration of stem cell platforms into preclinical pipelines supports steady demand for reproducible and scalable culture media.
Stem cell therapy manufacturing is projected to grow at the fastest rate of 14.89%, supported by the expansion of clinical-stage cell therapy programs and increasing focus on controlled upstream processing. Media optimized for expansion and differentiation under manufacturing conditions drive growth in this segment.
End-Use Insights
Pharmaceutical and biotechnology companies led the end-use segment with a share of 39.33%, supported by rising investment in stem cell research programs and internal cell therapy development pipelines. These organizations prioritize media systems that align with process standardization and batch-to-batch consistency.
CDMOs and CROs are expected to record the fastest growth at 14.46%, driven by the outsourcing of stem cell expansion, characterization, and manufacturing activities. Growing reliance on external service providers increases demand for adaptable culture media across diverse client projects.
End Use Market share (%) in 2025
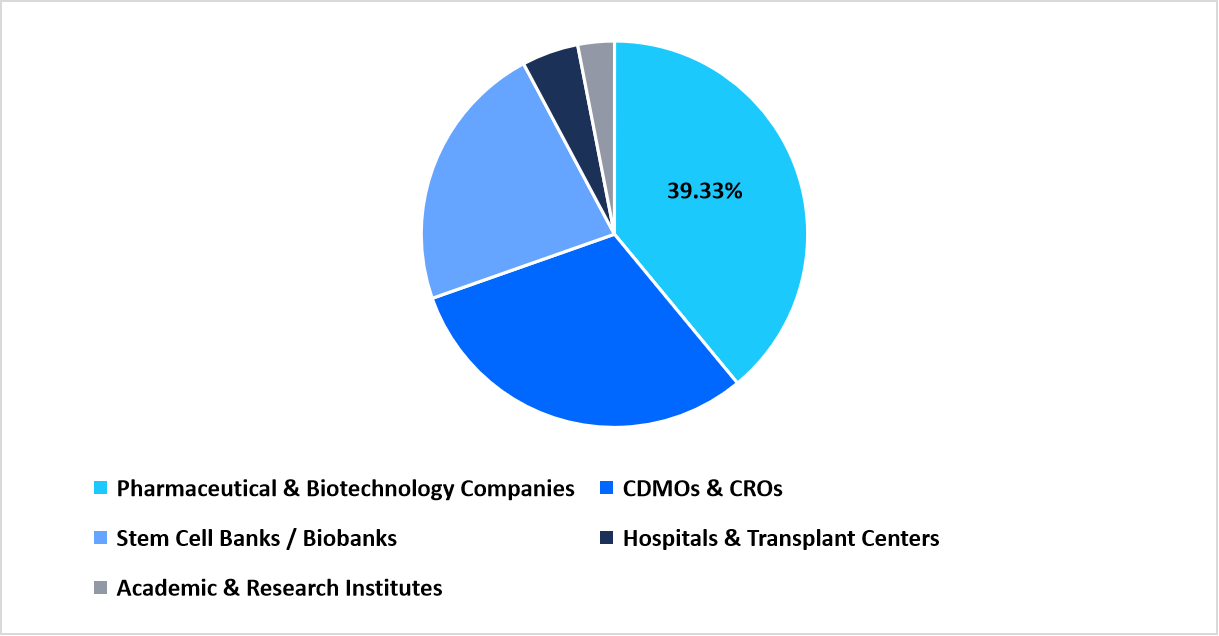
Source: Straits Research
Competitive Landscape
The global stem cell culture media market is moderately fragmented, with a mix of established life science suppliers and specialized cell culture media developers operating across research and clinical manufacturing environments.
Thermo Fisher Scientific Inc.: An Emerging Market Player
Thermo Fisher Scientific Inc. maintains a strong position in the stem cell culture media market through its broad portfolio of stem cell media and supplements designed for research and cell therapy workflows. The company supports culture systems for mesenchymal, hematopoietic, and pluripotent stem cells, enabling adoption across discovery and manufacturing settings. Its focus on standardized formulations and global distribution capabilities supports widespread use within pharmaceutical, biotechnology, and academic laboratories. Thermo Fisher continues to expand its cell culture offerings by aligning media development with evolving stem cell research and manufacturing requirements.
List of Key and Emerging Players in Stem Cell Culture Media Market
- Thermo Fisher Scientific Inc.
- Merck KGaA
- Cytiva
- Lonza Group AG
- Takara Bio Inc.
- STEMCELL Technologies Inc.
- FUJIFILM Irvine Scientific Inc.
- Corning Incorporated
- Miltenyi Biotec B.V. & Co. KG
- PromoCell GmbH
- Biological Industries Ltd.
- CellGenix GmbH
- PAN Biotech GmbH
- HiMedia Laboratories Pvt. Ltd.
- Sartorius AG
- Others
Strategic Initiatives
- May 2025: NextCell Pharma in Sweden entered a strategic collaboration with FUJIFILM Irvine Scientific, combining MSC products with optimized culture media and cryopreservation solutions to support stem cell therapy research.
- January 2025: Terumo Blood and Cell Technologies in the U.S. collaborated with FUJIFILM Irvine Scientific to accelerate T cell expansion using PRIME-XV T Cell Expansion Media, supporting scalable cell therapy manufacturing and research.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 757.30 Million |
| Market Size in 2026 | USD 856.35 Million |
| Market Size in 2034 | USD 2348.11 Million |
| CAGR | 13.44% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Cell Line, By Application, By End-use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Stem Cell Culture Media Market Segments
By Cell Line
- Mesenchymal Stem Cells (MSCs)
- Hematopoietic Stem Cells (HSCs)
- Induced Pluripotent Stem Cells (iPSCs)
- Embryonic Stem Cells (ESCs)
- Other Stem Cells
By Application
- Drug Discovery & Development
- Stem Cell Therapy Manufacturing
- Tissue Engineering & Regenerative Medicine
By End-use
- Pharmaceutical & Biotechnology Companies
- CDMOs & CROs
- Stem Cell Banks / Biobanks
- Hospitals & Transplant Centers
- Academic & Research Institutes
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































