Upstream Bioprocessing Market Size, Share & Trends Analysis Report By Product (Bioreactors/Fermenters, Cell Culture Products, Filters, Bioreactors Accessories, Bags & Containers, Others), By Usage (Multi-use, Single-use), By Mode (In-house, Outsourced) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Upstream Bioprocessing Market Size
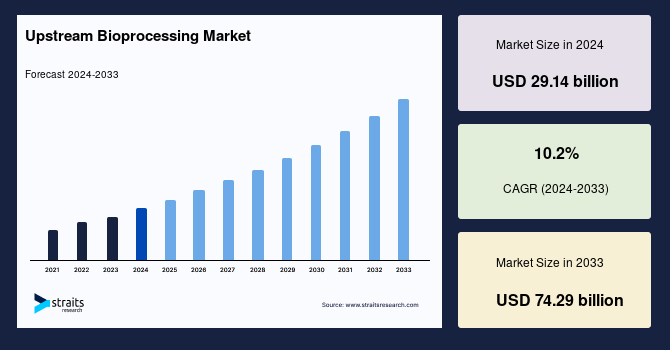
The global upstream bioprocessing market size was valued at USD 29.14 billion in 2024 and is projected to grow from USD 32.11 billion in 2025 to reach USD 69.84 billion in 2033, growing at a CAGR of 10.2% during the forecast period (2025–2033).
Upstream bioprocessing refers to the initial stages of the biomanufacturing process, where biological materials are cultivated and produced. This phase involves the use of living organisms, such as cells, to produce valuable products like vaccines, therapeutic proteins, and other biopharmaceuticals. The process typically begins with the selection of a suitable host organism, followed by cell culture, fermentation, and the optimization of environmental factors like temperature, pH, and nutrient supply to maximize the yield and quality of the desired product.
The market is witnessing substantial growth, driven by the increasing demand for biologics, including monoclonal antibodies, vaccines, cell and gene therapies, and recombinant proteins. A November 2024 article in Bioprocess Journal highlights a significant trend, citing data from the NIH and FDA, which reported that biologics accounted for approximately 30% of the 55 new drug approvals in 2023, a rise from the previous five-year average of about 28%. This surge in biologic approvals is prompting biopharmaceutical companies to focus more on improving the efficiency, scalability, and regulatory compliance of upstream processes.
To meet these demands, strategic collaborations between biopharmaceutical firms and technology providers are playing a key role in advancing upstream bioprocessing. Innovative solutions, such as perfusion-based bioprocessing and AI-driven process analytics, are being integrated to enhance yield and product quality. Moreover, the rise of biosimilars and personalized medicine is driving manufacturers to optimize their upstream processes for greater flexibility and scalability.
Continuous bioprocessing is also gaining attention for its ability to offer real-time monitoring and adaptive control of production parameters. A March 2023 study in Pharma Manufacturing revealed that over 40% of bioprocessing industry respondents identified continuous processing and perfusion as key areas to be tested or evaluated in the upcoming year. With sustained investments in research and development, as well as advancements in biomanufacturing infrastructure, the market is positioned for continued growth. This growth will be essential in meeting the evolving needs of the rapidly expanding sector.
Latest Market Trends
Single-Use Technologies Transforming Upstream Bioprocessing
The growing shift towards single-use technologies in upstream bioprocessing is revolutionizing the industry due to their flexibility, cost-effectiveness, and reduced risk of cross-contamination. Disposable bioreactors, media bags, and filtration systems minimize cleaning and validation requirements, accelerating turnaround time. As a result, companies are increasingly investing in scalable single-use platforms for faster biologics production.
- For example, in April 2023, Cytiva launched the Xcellerex X-platform bioreactors, designed to streamline upstream bioprocessing by enhancing modular single-use capabilities for commercial-scale manufacturing.
This aligns with the increasing demand for disposable, cost-effective solutions in biologics manufacturing, further driving market growth.
Automation and Digitalization Enhancing Bioprocess Efficiency
Automation and digitalization are transforming upstream bioprocessing by improving process control, data analytics, and reproducibility. AI-driven bioprocess modeling, real-time monitoring, and automated feeding strategies are optimizing cell culture conditions, boosting yields, and improving product consistency.
- For instance, in February 2025, Cultivated B launched a new line of multi-channel biosensors designed to enhance the monitoring of cell culture and fermentation processes in biomanufacturing. These are integrated with artificial intelligence (AI) to offer real-time data analytics, providing bioprocess engineers with tools to improve accuracy and decision-making in bioprocessing.
Such automation is enabling biomanufacturers to shift toward smart factories, where automated control systems drive efficiency, minimize human error, and ensure consistent biologics production at an industrial scale.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 29.14 Billion |
| Estimated 2025 Value | USD 32.11 Billion |
| Projected 2033 Value | USD 69.84 Billion |
| CAGR (2025-2033) | 10.2% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Thermo Fisher Scientific, Inc., Merck KGaA, Corning Incorporated, Sartorius AG, Eppendorf SE |
to learn more about this report Download Free Sample Report
Upstream Bioprocessing Market Growth Factors
Expansion of Cell and Gene Therapy Manufacturing
The increasing demand for cell and gene therapies is accelerating advancements in upstream bioprocessing, particularly in scalable bioreactor systems and optimized cell culture media. As the demand for biologics, including cell and gene therapies, grows, manufacturing facilities are expanding to meet this need.
- For example, in October 2022, Lonza expanded its cell and gene therapy manufacturing facility in Geleen, Netherlands, to enhance viral vector production.
This expansion reflects the growing need for efficient upstream bioprocessing solutions that can support the rising demand for advanced biologics, driving further market growth.
Strategic Partnerships Accelerating Bioprocess Innovation
Collaborations between biopharmaceutical firms and technology providers are accelerating innovations in upstream bioprocessing, particularly in bioreactor design, automation, and cell culture optimization. These partnerships are key in driving advancements that improve biomanufacturing processes to meet growing demand.
- For instance, in September 2024, Multiply Labs and Legend Biotech announced a collaboration to automate cell therapy manufacturing using advanced robotics. By leveraging their combined expertise, these companies are advancing upstream processing technologies to support the production of biologics, thus stimulating further demand for biomanufacturing solutions.
Market Restraining Factor
High Capital Costs for Advanced Bioprocessing Technologies
The high capital costs associated with advanced upstream bioprocessing technologies are a significant restraint in the market. Technologies such as AI-driven automation, high-end perfusion bioreactors, and real-time monitoring systems require substantial investment, which can be challenging for smaller biotech firms. This financial burden limits their ability to scale production efficiently.
Moreover, the high costs of implementing Process Analytical Technology (PAT) and single-use bioreactors add to these constraints. Many emerging biopharma companies face difficulties securing funding to invest in state-of-the-art upstream processing infrastructure, hindering their ability to compete and meet growing demand in the biologics sector.
Market Opportunity
Expansion of Modular and Prefabricated Bioprocessing Facilities
The rising demand for flexible and scalable biomanufacturing has driven the adoption of modular and prefabricated bioprocessing facilities. These facilities offer rapid deployment, cost savings, and compliance with GMP standards, making them ideal for biologics and gene therapy production.
- For example, Cytiva expanded its KUBio modular facility solutions to support advanced biomanufacturing. These facilities enable companies to quickly deploy new manufacturing capabilities, reducing facility setup times from years to months.
Such developments create lucrative opportunities for biopharma companies to quickly scale production while maintaining regulatory compliance, reducing facility setup times from years to months, and addressing the global demand for high-quality biologics.
Regional Insights
North America remains a dominant force in the global upstream bioprocessing market, bolstered by its robust biopharmaceutical ecosystem, substantial R&D investments, and cutting-edge manufacturing infrastructure. The region is home to a high concentration of biologics manufacturers, with major players like Amgen, Pfizer, and Moderna leading the charge in innovation. Key biotech hubs, such as Boston and California’s Bay Area, facilitate collaborative research and process development. Moreover, government support, including the U.S. FDA’s initiatives to advance biomanufacturing and the NIH’s funding for cell and gene therapy programs, strengthens North America’s position as the market leader.
The U.S. leads the market, driven by strong government initiatives and a well-established biopharma ecosystem. The Biden administration’s $2 billion biomanufacturing initiative, announced in September 2022, is accelerating domestic biologics production and innovation. This funding supports advancements in bioprocessing infrastructure, workforce training, and next-generation technologies like continuous bioprocessing. Moreover, the presence of major biotech hubs, including Boston and California, fosters collaboration among industry leaders such as Amgen and Regeneron, further strengthening the country’s dominance.
Asia Pacific Upstream Bioprocessing Market Trends
Asia-Pacific is poised to register the highest CAGR in the global upstream bioprocessing market, driven by rapid biopharma expansion and strategic investments in biologics manufacturing. Countries such as China, India, and South Korea are significantly increasing their bioprocessing capacities, supported by government policies like China’s “Made in China 2025” and India’s Biopharma Mission. The growing presence of contract development and manufacturing organizations (CDMOs) like WuXi Biologics and Samsung Biologics, along with lower operational costs, further fuel the region's market growth.
South Korea has emerged as a biomanufacturing powerhouse, with CDMOs like Samsung Biologics and SK Bioscience leading global upstream bioprocessing. Samsung Biologics operates the world’s largest biopharma manufacturing site, with roughly 700,000 Liters of bioreactor capacity. Additionally, government support, such as the “K-Bio” initiative, is fostering innovation in upstream technologies. The country’s expertise in hybrid bioprocessing, integrating stainless steel and single-use systems, is enhancing flexibility in large-scale biologics production.
Europe Upstream Bioprocessing Market Trends
Germany is a key hub for upstream bioprocessing, supported by its strong biologics manufacturing ecosystem and advanced engineering capabilities. The country has over 250 biopharma production sites, with leading firms like Merck KGaA and Sartorius driving innovation in bioreactor technology and automation. Government-backed initiatives are promoting the adoption of single-use bioreactors and continuous bioprocessing, enhancing production efficiency. Germany’s highly skilled workforce and robust infrastructure make it a crucial player in global upstream biomanufacturing advancements.
The UK’s market is bolstered by strong academic-industry collaboration and advanced biomanufacturing clusters like the Oxford-Cambridge-London triangle. The country is a leader in cell and gene therapy production, with the UK Cell and Gene Therapy Catapult supporting bioprocess scale-up. In May 2023, the UK government announced a £650 million investment in life sciences manufacturing, enhancing capabilities in upstream processing for novel biologics. The country’s emphasis on automation and AI-driven bioprocessing further strengthens its competitive edge.
France is strengthening its position in upstream bioprocessing through major biomanufacturing investments and government support. Sanofi’s €1 billion investment in France, announced in May 2024, focuses on expanding biologics production, including monoclonal antibodies and recombinant therapies. The country also benefits from initiatives such as France’s “Innovation Santé 2030” program, which aims to boost biomanufacturing capacity and innovation. With a strong pharmaceutical sector and growing adoption of modular bioprocessing technologies, France is emerging as a key player in next-generation biologics production.
Product Insights
The bioreactors/fermenters segment leads the market for upstream bioprocessing, capturing the highest revenue due to their crucial role in cell culture expansion, microbial fermentation, and high-yield biologics production. As demand for monoclonal antibodies, cell and gene therapies, and recombinant proteins rises, the adoption of both single-use and stainless-steel bioreactors has surged.
- For instance, in September 2023, Getinge launched a single-use bioreactor designed for clinical cell and gene therapy manufacturing, reinforcing the dominance of bioreactors in upstream bioprocessing.
Usage Insights
The multi-use segment holds the largest market share in upstream bioprocessing, favored for its durability, scalability, and cost-effectiveness in large-scale biologics production. Stainless steel-based multi-use systems provide excellent process control, sterility assurance, and can handle high-volume production, making them ideal for monoclonal antibody and recombinant protein manufacturing. Their versatility and proven performance in maintaining consistent product quality make them the go-to choice for biopharmaceutical companies seeking to meet the growing demand for complex biologics efficiently.
Mode Insights
The in-house segment dominates the global market, driven by biopharmaceutical companies seeking greater control over production quality, scalability, and intellectual property. Owning in-house manufacturing facilities allows firms to streamline operations, optimize processes, ensure strict regulatory compliance, and safeguard proprietary technologies. This autonomy minimizes reliance on contract manufacturers and fosters innovation, making in-house production the preferred choice for companies aiming for long-term success in biologics manufacturing.
Company Market Share
Key players in the industry are increasingly focusing on implementing a range of strategic business initiatives to strengthen their position in the market. These include forging strategic collaborations, securing product approvals, pursuing acquisitions, and launching innovative products to drive growth and expand their market share. By aligning with leading technology providers, investing in research and development, and acquiring complementary businesses, companies aim to accelerate their product offerings and enhance operational capabilities.
Samsung Biologics: An Emerging Player in the Global Upstream Bioprocessing Market
Samsung Biologics is a leading biopharmaceutical company specializing in the manufacturing of biologic drugs, with expertise in developing and commercializing biopharmaceutical products. The company offers end-to-end solutions for biologics, from cell line development and process optimization to large-scale manufacturing, focusing on monoclonal antibodies, vaccines, and cell and gene therapies.
Recent Developments by Samsung Biologics:
- In March 2023, Samsung Biologics unveiled a new single-use bioreactor facility at its South Korean production site, expanding its upstream bioprocessing capacity. This development is aimed at supporting the growing demand for monoclonal antibodies and gene therapies.
List of Key and Emerging Players in Upstream Bioprocessing Market
- Thermo Fisher Scientific, Inc.
- Merck KGaA
- Corning Incorporated
- Sartorius AG
- Eppendorf SE
- Danaher
- Boehringer Ingelheim International GmbH
- Getinge
- PBS Biotech, Inc.
- Lonza
- VWR International, LLC
- Meissner Filtration Products, Inc.
- Repligen Corporation
- Entegris
- Kuhner AG

to learn more about this report Download Market Share
Recent Developments
- February 2025 – Thermo Fisher Scientific announced the acquisition of Solventum's purification and filtration division for approximately $4.1 billion. This strategic move aims to enhance Thermo Fisher's position in the bioprocessing filtration sector, enabling the company to better compete with industry leaders like Danaher and Repligen.
Analyst Opinion
As per our analyst, the global upstream bioprocessing market is poised for substantial growth, fueled by the increasing demand for biologics, cell and gene therapies, and the ongoing advancements in biomanufacturing technologies. Strategic partnerships between biopharmaceutical firms and technology providers, coupled with government initiatives supporting biomanufacturing innovation, are further accelerating market development.
Despite these positive trends, challenges such as high capital investment requirements, regulatory hurdles, and the need for specialized expertise remain. Smaller biotech firms, in particular, struggle with securing the necessary funding to adopt cutting-edge technologies, which can limit their growth potential.
However, with the increasing approval of new biologics and advancements in process optimization, the market continues to expand. Companies are focusing on improving production capacity and streamlining upstream processes, ensuring that the market will continue to evolve in response to the growing demand for advanced therapies and more efficient manufacturing solutions.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 29.14 Billion |
| Market Size in 2025 | USD 32.11 Billion |
| Market Size in 2033 | USD 69.84 Billion |
| CAGR | 10.2% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Usage, By Mode |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Upstream Bioprocessing Market Segments
By Product
- Bioreactors/Fermenters
- Cell Culture Products
- Filters
- Bioreactors Accessories
- Bags & Containers
- Others
By Usage
- Multi-use
- Single-use
By Mode
- In-house
- Outsourced
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































