Veterinary Analgesics and Anti-Inflammatory Market Size, Share & Trends Analysis Report By Drug Class (Non-steroidal anti-inflammatory drugs (NSAIDs), Opioids and partial agonists, Local anaesthetics/nerve blocks, Corticosteroids, Biologics / monoclonal antibodies, Adjunctive analgesics, Topical analgesics/counterirritants), By Route of Administration (Oral solids, Oral liquids, Injectable, Transdermal/topical, Implantable / depot formulations, Ophthalmic/local formulations), By Animal Class (Companion animals, Equine, Production/food animals, Laboratory/research animals), By Indication (Postoperative/perioperative pain control, Chronic musculoskeletal pain, Acute trauma/injury, Procedural analgesia, Topical/localised pain), By Distribution Channel (Veterinary hospitals and clinics, Veterinary retail pharmacies and wholesalers, Online pharmacies, Farm supply/dealer channels) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Veterinary Analgesics and Anti-Inflammatory Market Overview
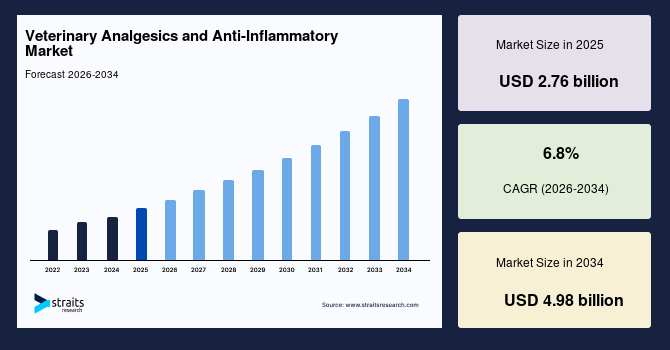
The global veterinary analgesics and anti-inflammatory market size was estimated at USD 2.76 billion in 2025 and is anticipated to grow from USD 2.94 billion in 2026 till USD 4.98 billion by 2034, growing at a CAGR of 6.8% from 2026-2034. The global market growth is attributed to the rising pet ownership and higher per-pet veterinary spend, the ageing pet population with chronic pain needs, expanded clinical guidelines for peri-operative and chronic pain management, and a wave of novel modalities.
The veterinary analgesics and anti-inflammatory market encompasses NSAIDs, opioids/partial agonists, monoclonal antibodies, and adjunctive therapies used for acute surgical pain, chronic musculoskeletal pain, and inflammatory conditions across companion animals and livestock. Regulatory and welfare pressures, particularly in developed markets, increase routine pain management in production animals and companion care.
Key Market Trends & Insights
- North America held a dominant share of the global market with a market share of 32.2% in 2025, owing to high veterinary spend per household, large companion-animal population, and rapid uptake of novel biologics.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 8.4%, due to rising pet ownership, expanding vet services and growing livestock production.
- Based on animal class, the companion animals segment is estimated to grow at a CAGR of 7.2%.
- Based on the indication, chronic musculoskeletal pain will hold a market share of 46.5% in 2025.
- The U.S. dominates the market in 2025.
Market Revenue Figures
- 2025 Market Size: USD 2.76 billion
- 2034 Projected Market Size: USD 4.98 billion
- CAGR (2026-2034): 6.8%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
Latest Market Trends
Biological and long-acting therapies reshape chronic pain care
Veterinary medicine is shifting away from only short-acting drugs like NSAIDs or regular opioids toward long-acting treatments and biologics. Biologics offer targeted mechanisms with monthly dosing that align with owner convenience and provide economic benefits.
- For example, Zoetis’ Librela (bedinvetmab), a monthly monoclonal antibody injection for dogs, was approved by the U.S. FDA to treat osteoarthritis pain in May 2023. In March 2025, a head-to-head study showed Librela had pain relief similar to meloxicam (an NSAID) but with fewer side effects.
These developments make long-acting treatments more trusted by veterinarians and pet owners, improving convenience and fewer clinic visits with potentially better adherence.
Protocolisation of pain management and multimodal care in clinics
Veterinary doctors increasingly follow formal protocols for pain relief, using more than one approach together, like combining NSAIDs, adjunct medicines (e.g., non-NSAID pain modulators), physical therapy, and better owner education. This trend is driven by professional guidelines, continuing education, and veterinarians’ quality-of-care metrics.
- For example, 2024 AVMA data show pet-owner visits for chronic conditions (such as osteoarthritis) increasing, which encourages clinics to adopt protocols that include diagnostics, regular follow-ups, and combination therapy.
These standardised approaches reduce variation in care and help clinics plan and predict the use of premium therapies.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 2.76 billion |
| Estimated 2026 Value | USD 2.94 billion |
| Projected 2034 Value | USD 4.98 billion |
| CAGR (2026-2034) | 6.8% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Zoetis, Elanco, Merck Animal Health (MSD), Boehringer Ingelheim Animal Health, Dechra |
to learn more about this report Download Free Sample Report
Market Drivers
Regulatory and welfare standards are pushing wider analgesic use
Governments and veterinary bodies worldwide are strengthening rules and expectations around animal welfare. This includes requiring better pain relief during routine farm procedures (like castration and dehorning) and surgery in companion animals.
- For instance, in June 2025, Western Australia passed the Veterinary Practice Amendment Regulations 2025, which explicitly cover veterinary anaesthesia and analgesia standards.
These developments make it more likely that analgesic medications, including anti-inflammatories and biologics, are endorsed, approved, and demanded in both companion-animal and production-animal sectors.
Companion-animal population growth and increasing veterinary expenditure
Rising pet ownership and ‘pet humanization’ remain foundational drivers of the market. Higher household pet penetration and willingness to spend on healthcare translate into larger addressable markets for analgesics and supportive therapies.
- According to the AVMA data, the dog population has steadily increased to a new peak of 89.7 million in 2024, and the cat population has reached 73.8 million in 2024. This has resulted in rising veterinary visits for chronic conditions like osteoarthritis.
Manufacturers and distributors are seeing a growth potential, justifying R&D in higher-value products like biologics and expanding clinical support programs.
Market Restraint
Owner cost sensitivity and clinic capacity constraints
Despite the availability of better pain treatments, many pet owners or farmers cannot afford them, especially for long-acting biologics or repeated follow-ups. Clinics often have limited time and staff to monitor chronic pain or administer premium treatments. For example, the high cost of biologics and long-acting therapies means some owners defer or decline them. Also, in rural or low-income regions, veterinary workforce shortages or fewer specialty clinics reduce access. Due to these cost and capacity constraints, adoption of new premium therapies is slower in many markets.
Market Opportunity
Long-acting formulations and innovations in delivery
There is a strong growth opportunity in delivery methods that reduce dosing frequency and improve compliance.
- For example, Elanco’s Zorbium (buprenorphine transdermal solution) for cats provides four days of postoperative pain control with a single in-clinic application, reducing the need for repeated dosing at home.
- Also, a 2025 study in PMC demonstrated the analgesic efficacy of a long-acting transdermal buprenorphine formulation, confirming that extended pain relief doesn’t compromise safety.
Such long-acting products are especially beneficial where frequent veterinary access is difficult, owners may miss doses, or stress or handling risk is high. These innovations help expand the market both in companion and production animals by offering more convenient, safer, and better-adhered treatments.
Regional Analysis
North America’s market dominance is driven by high pet ownership numbers, strong per-household veterinary spending, and a dense clinical and specialty hospital network that adopts novel therapeutics rapidly. The AVMA (2024) reports nearly 90 million dogs and 74 million cats in the U.S., creating a vast customer base. Regulatory clarity from the FDA Center for Veterinary Medicine supports timely approvals but also enforces rigorous post-market monitoring. Clinics in the U.S. and Canada are early adopters of subscription models for monthly biologics and bundled rehabilitation services. Strong welfare standards and high household expenditure ensure North America remains the dominant market.
Asia Pacific - Fastest-growing region
The Asia Pacific region is the fastest-growing market, driven by rapid urbanization, rising pet ownership, and government initiatives in animal health. China and India have seen large increases in veterinary infrastructure, with expanding clinics and rising household spending on pets. In 2024, India’s Standard Veterinary Treatment Guidelines included welfare-linked pain management protocols, promoting adoption in livestock as well. While affordability challenges remain, especially in production animals, demand for cost-effective long-acting injectables is rising. Global companies are accelerating local product filings and partnerships in the Asia Pacific, recognizing its dual growth track of mass-market livestock demand and a rapidly expanding companion-animal premium segment.
Country Insights
U.S. Market Trends
The U.S. is the largest market for veterinary analgesics, supported by high pet ownership and strong household spending on pet healthcare. According to the AVMA, there are about 89.7 million dogs and 73.8 million cats in the country, driving steady demand for chronic pain management (e.g., osteoarthritis) and peri-operative analgesia. Clinics adopt premium therapies such as biologics and long-acting injectables quickly, supported by a strong clinical infrastructure, supporting overall market growth in the U.S.
Canada Market Trends
Canada’s veterinary analgesics market is driven by rising pet ownership, higher household spending, and strong professional guidance from the Canadian Veterinary Medical Association (CVMA). Companion-animal demand is growing steadily, though adoption of high-cost biologics varies by region due to clinic access and owner affordability. Government emphasis on animal welfare and transparent pricing discussions is shaping future adoption. Flexible pricing strategies and clinic education programs are key tools for manufacturers to strengthen market penetration.
Germany's Market Trends
Germany has a strong veterinary market supported by high pet ownership and animal welfare laws embedded in the constitution. The Animal Welfare Act requires humane care, driving adoption of pain-relief products in both companion and production animals. Companion-animal clinics are well equipped and quick to adopt premium biologics and injectables, especially for osteoarthritis care. Germany’s large e-commerce sector also supports the distribution of NSAIDs and topical products. Regulatory scrutiny and NGO advocacy keep analgesic use on the policy agenda, sustaining steady market growth.
China Market Growth Factors
China is one of the fastest-growing veterinary analgesics market, driven by rapid urbanization and rising disposable incomes. Younger pet owners are more willing to pay for advanced healthcare, fueling demand for osteoarthritis treatments and peri-operative analgesia. Government initiatives to modernize veterinary regulation and welfare standards support expansion in both companion and production animals. Multinationals are investing in local partnerships and registrations, while demand for long-acting injectables is increasing in farms to meet welfare and compliance needs.
Indian Market Trends
India’s veterinary analgesics market is shaped by its dual focus: a massive livestock population and growing companion-animal demand in cities. The Department of Animal Husbandry and Dairying (DAHD) published Standard Veterinary Treatment Guidelines in October 2024, which encourage formal pain management in farm animals. In urban centers, rising pet ownership and new private clinics are increasing demand for osteoarthritis and chronic-care therapies.
Drug Class Insights
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most widely used analgesics in veterinary medicine. They are effective for post-surgical pain, arthritis, and inflammatory conditions in dogs, cats, horses, and farm animals. Their growth is supported by affordability, well-documented safety, and strong clinician familiarity. Many veterinary guidelines recommend NSAIDs as first-line therapy, making them routine in clinics and farms. Their broad approvals, ease of dosing, and low regulatory complexity compared to opioids ensure they remain the leading drug class.
Route of Administration Insights
Injectable formulations, especially long-acting depot injections, are growing rapidly because they provide reliable dosing and reduce the risk of owner error. In clinics, they are favored for surgeries and chronic conditions where monthly biologics require professional administration. For farm animals, long-acting injections reduce handling frequency, aligning with welfare-driven practices. As regulatory approvals expand for injectable biologics, veterinarians increasingly prefer them over oral options, despite higher cost, because they combine effectiveness, convenience, and compliance benefits for both owners and animals.
Animal Class Insights
Companion animals like dogs and cats account for most veterinary analgesic sales because owners are willing to spend more on their care compared to livestock. Ageing pet populations are increasing cases of osteoarthritis, a condition requiring ongoing pain management. Owners often view pets as family members, which boosts demand for advanced treatments such as monthly monoclonal antibodies or long-acting transdermal patches. Clinics adopt standard pain protocols and prefer clinic-administered therapies, ensuring consistent prescribing.
Indication Insights
Chronic musculoskeletal disorders, particularly osteoarthritis in ageing dogs and cats, represent the largest therapeutic need. Osteoarthritis requires lifelong treatment and regular veterinary monitoring, creating steady demand for analgesics. Historically, NSAIDs dominated, but new biologics now offer monthly alternatives that generate higher revenue per patient. Veterinary guidelines increasingly recommend early diagnosis and multimodal therapy, combining drugs with physiotherapy. This structured approach ensures chronic pain remains the most important driver of prescriptions and long-term market value.
Distribution Channel Insights
Veterinary hospitals and clinics are the main distribution points for analgesics. Many high-value therapies, such as biologics, controlled opioids, and depot injectables, require veterinary supervision. Clinics administer pain control during surgeries, provide monthly injections for osteoarthritis, and dispense products like Zorbium transdermal patches for cats. Clinics also benefit financially from recurring visits tied to pain management. Manufacturers support this channel by training veterinarians and offering clinic programs, ensuring that veterinary practices remain the central hub for analgesic distribution worldwide.
Competitive Landscape
The global market is highly fragmented, dominated by large animal-health companies alongside regional specialists. Large players in premium biologics, NSAIDs, and subscription models. Mid-sized firms emphasize niche formulations and fast regional launches. Distribution-focused players and online pharmacies increase access to NSAIDs and topicals. Education, clinic partnerships, and pharmacovigilance remain core strategies across the sector.
Zoetis - A Market Leader
Zoetis leads the veterinary analgesics market with a broad portfolio of biologics and NSAIDs. Its strategy centers on clinic-administered premium therapies and post-market safety monitoring. The company’s Librela® (bedinvetmab) is the first monoclonal antibody approved for canine osteoarthritis pain and has quickly become a flagship product.
Latest News:
- In February 2025, Zoetis announced a U.S. label update for Librela, reflecting safety data and strengthening clinician confidence.
List of Key and Emerging Players in Veterinary Analgesics and Anti-Inflammatory Market
- Zoetis
- Elanco
- Merck Animal Health (MSD)
- Boehringer Ingelheim Animal Health
- Dechra
- Ceva Santé Animale
- Vetoquinol
- Virbac
- Bayer Animal Health
- Norbrook
- Phibro
- Hipra
- Ceva
- Bayer/Elanco
- Kyoritsu
Recent Developments
- May 2025- Merck Animal Health announced an $895 million expansion of its manufacturing and R&D facilities in De Soto, Kansas. This is a significant investment aimed at increasing the company's biologics and vaccine production capacity.
- May 2025- Dechra announced FDA approval of Otiserene (marbofloxacin, terbinafine, and dexamethasone otic suspension). This product is a single-dose, long-acting treatment for otitis externa (ear infections) in dogs.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 2.76 billion |
| Market Size in 2026 | USD 2.94 billion |
| Market Size in 2034 | USD 4.98 billion |
| CAGR | 6.8% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Drug Class, By Route of Administration, By Animal Class, By Indication, By Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Veterinary Analgesics and Anti-Inflammatory Market Segments
By Drug Class
- Non-steroidal anti-inflammatory drugs (NSAIDs)
- Opioids and partial agonists
- Local anaesthetics/nerve blocks
- Corticosteroids
- Biologics / monoclonal antibodies
- Adjunctive analgesics
- Topical analgesics/counterirritants
By Route of Administration
- Oral solids
- Oral liquids
- Injectable
- Transdermal/topical
- Implantable / depot formulations
- Ophthalmic/local formulations
By Animal Class
- Companion animals
- Equine
- Production/food animals
- Laboratory/research animals
By Indication
- Postoperative/perioperative pain control
- Chronic musculoskeletal pain
- Acute trauma/injury
- Procedural analgesia
- Topical/localised pain
By Distribution Channel
- Veterinary hospitals and clinics
- Veterinary retail pharmacies and wholesalers
- Online pharmacies
- Farm supply/dealer channels
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































