Veterinary Antimicrobial Susceptibility Testing Market Size, Share & Trends Analysis Report By Product (2026-2034) (Disks & Plates, Culture Media, Accessories & Consumables, Automated AST Instruments), By Animal Type (2026-2034) (Production Animal, Cattle, Poultry, Pigs, Others, Companion Animal, Dogs, Cats, Horses), By End Use (2026-2034) (Veterinary Reference Lab, Vet Research Institutes, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Veterinary Antimicrobial Susceptibility Testing Market Overview
The global veterinary antimicrobial susceptibility testing market size is valued at USD 1.22 billion in 2025 and is estimated to reach USD 2.43 billion by 2034, growing at a CAGR of 7.97% during the forecast period. The consistent market growth is supported by the mandatory integration of bacterial isolation libraries with veterinary AST platforms, enabling longitudinal resistance mapping and incentivizing testing.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 35.11% in 2025.
- The Asia Pacific region is projected to grow at the fastest pace, with a CAGR of 9.42% during the forecast timeframe.
- Based on product, the automated AST instruments segment dominated the market with a revenue share of 42.94% in 2025.
- By animal type, the companion animal segment is expected to register the fastest CAGR of 8.14%. during the forecast timeframe.
- Based on end use, the vet research institutes segment is estimated to register the fastest CAGR of 8.69% during the forecast timeframe.
- S. dominates the veterinary antimicrobial susceptibility testing market, valued at USD 360.79 million in 2024 and reaching USD 387.99 million in 2025.
Table: U.S. Veterinary Antimicrobial Susceptibility Testing Market Size (USD Million)
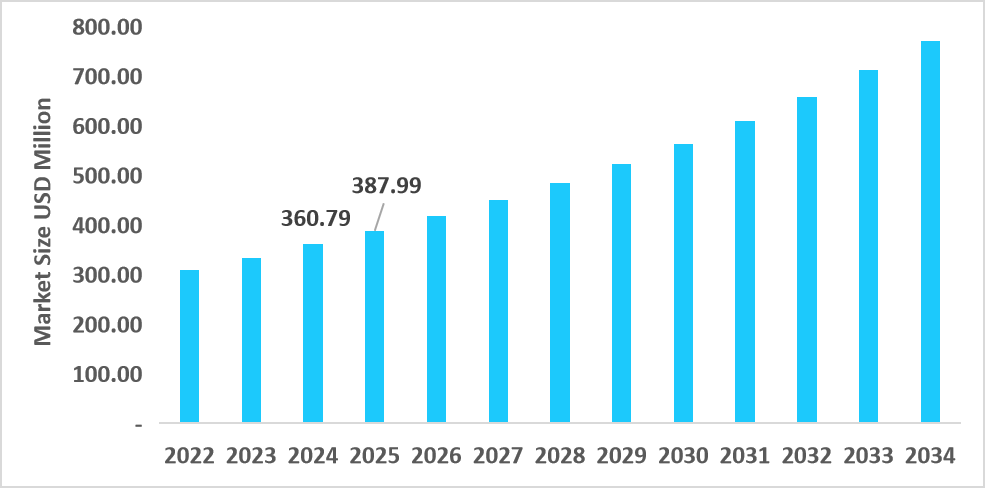
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.22 billion
- 2034 Projected Market Size: USD 2.43 billion
- CAGR (2026-2034): 7.97%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The veterinary antimicrobial susceptibility testing market encompasses diagnostic solutions used to determine the effectiveness of antimicrobial agents against animal pathogens. It includes products such as disks and plates, culture media, consumables, and automated AST instruments, applied across production animals, including cattle, poultry, and pigs, as well as companion animals. These solutions are utilized by veterinary reference laboratories, research institutes, and other animal health facilities.
Latest Market Trends
Expansion of Point-of-Care Rapid AST Platforms
A major emerging trend in the veterinary antimicrobial susceptibility testing market is the rapid expansion and adoption of point-of-care AST solutions that deliver actionable susceptibility results within hours directly at veterinary clinics, reducing reliance on centralized reference labs for faster antimicrobial decisions and stewardship in both production and companion animal care. These rapid systems, such as miniAST, are reshaping diagnostic workflows and accelerating clinical response times.
Standardization of EUCAST/CLSI Harmonized Testing Protocols
The widespread adoption of globally harmonized AST standards such as EUCAST and CLSI VET protocols across veterinary laboratories is a major trend for market growth. These standards enhance comparability of susceptibility results, support regulatory compliance, and streamline antimicrobial stewardship initiatives. This shift toward uniform testing frameworks is encouraging equipment upgrades, expanding reference lab networks, and driving investment in standardized consumables and automated systems worldwide.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.22 Billion |
| Estimated 2026 Value | USD 1.31 Billion |
| Projected 2034 Value | USD 2.43 Billion |
| CAGR (2026-2034) | 7.97% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Thermo Fisher Scientific, bioMérieux, BD, Bio-Rad Laboratories Inc., Bruker Corporation |
to learn more about this report Download Free Sample Report
Market Drivers
Escalating Demand for Quantitative and Rapid AST Solutions in Intensive Farming
The increasing demand for quantitative and rapid AST technologies within intensive livestock operations to curb production losses from infectious outbreaks is a key driver for market growth. For instance, major integrators in the poultry and swine sectors are investing in on-farm rapid AST platforms that provide MIC values in hours, enabling precise antimicrobial selection and reduced mortality. This operational requirement is prompting broader procurement of advanced AST systems across production animal facilities.
Market Restraints
High Cost and Maintenance of Automated AST Platforms
The substantial capital investment and ongoing maintenance costs associated with automated AST instruments deter smaller veterinary clinics and diagnostic centers from adoption. Many manufacturers note in financial reports that high upfront expenses for equipment, software upgrades, and service contracts limit procurement to larger reference labs, slowing market penetration in decentralized veterinary practices and cost-sensitive regions.
Market Opportunity
Integration of AST Data with National AMR Surveillance Programs
A key opportunity in the veterinary antimicrobial susceptibility testing market lies in leveraging AST data integration with national antimicrobial resistance surveillance systems, for resistance tracking across animal populations. Major diagnostics firms emphasize collaborations with government agencies to feed AST results into platforms like the U.S. National Antimicrobial Resistance Monitoring System (NARMS), enhancing epidemiological insights, informing policy decisions, and expanding demand for standardized, connected testing solutions.
Regional Analysis
North America dominated the veterinary antimicrobial susceptibility testing market in 2025, accounting for 35.11% market share. The growth is supported by the prevalence of federally funded AMR veterinary research consortia that mandate participation in inter-laboratory AST proficiency testing programs, ensuring diagnostic quality benchmarking, accelerating uptake of standardized AST methods, and driving sustained high testing volumes across veterinary reference labs and academic partners.
Canada's market growth is driven by the implementation of a national veterinary antimicrobial sales reporting system that quantifies antimicrobial availability by animal species and informs stewardship strategies, enhancing data intelligence, regulatory compliance, and targeted AST utilization in animal health surveillance.
Asia Pacific Market Insights
Asia Pacific is emerging as the fastest-growing region with a CAGR of 9.42% from 2026 to 2034. This growth is augmented by the emergence of integrated livestock export compliance programs in countries like China and Australia that mandate AST data submission to meet stringent food safety standards for international meat and animal product shipments, driving routine laboratory testing and diagnostic infrastructure investments.
In Australia, the market growth is supported by the nation’s National Animal Health Diagnostics Business Plan, driving the establishment of a dedicated national AMR reference laboratory with standardized AST procedures and innovative diagnostics validation, strengthening diagnostic capacity, surveillance integration, and adoption of high-quality susceptibility testing across state and territory veterinary labs.
Regional Market share (%) in 2025
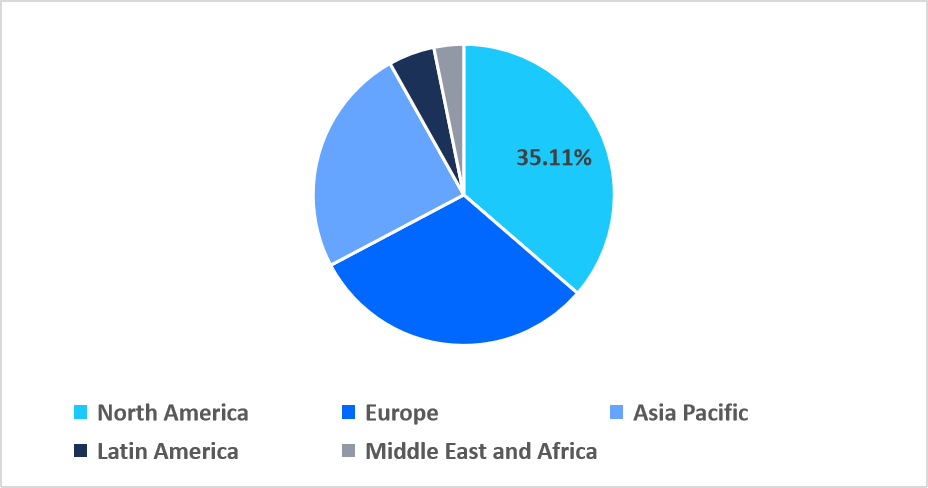
Source: Straits Research
Europe Market Insights
Europe’s veterinary antimicrobial susceptibility testing market is experiencing strong growth due to the establishment and expansion of the European Antimicrobial Resistance Surveillance network in Veterinary Medicine, a dedicated EU-wide AMR monitoring system harmonizing AST methods, generating comparable resistance data, and guiding region-specific treatment guidelines.
In Germany, the market growth is driven by the nation’s GERM-Vet national resistance monitoring program, which coordinates 30 labs to systematically collect and test animal pathogen isolates against standardized antibiotic panels, generating robust resistance data that directly informs veterinarians’ treatment choices and increases routine AST utilization.
Latin America Market Insights
Latin America’s veterinary antimicrobial susceptibility testing market is growing steadily due to the increasing emphasis by export-oriented livestock producers on standardized AST as a prerequisite for international meat and poultry trade certifications, compelling diagnostic laboratories to adopt robust susceptibility testing protocols to meet stringent food safety and export requirements.
Argentina's veterinary antimicrobial susceptibility testing market is expanding rapidly due to the country’s enforcement of strict AMR regulations that prohibit critical antimicrobial uses in livestock, such as the ban on colistin and growth-promoting antibiotics under national AMR strategy policies, prompting producers to increasingly rely on formal AST data to justify therapeutic antibiotic use and comply with surveillance requirements.
Middle East and Africa Market Insights
The Middle East and Africa veterinary antimicrobial susceptibility testing market growth is propelled by the expansion of structured hands-on AMR diagnostic capacity building programs, such as advanced microbiology training workshops for East African veterinary lab personnel that strengthen regional AST competencies and elevate local testing quality and usage rates. This capacity development boosts the adoption of susceptibility testing across underserved veterinary networks.
South Africa's veterinary antimicrobial susceptibility testing market is witnessing growth driven by the strengthening of laboratory quality frameworks through accreditation by the South African National Accreditation System (SANAS), which compels veterinary diagnostic labs to achieve ISO/IEC 17025 standards for AST competency, thereby driving demand for advanced AST services.
Product Insights
The automated AST instruments segment dominated the market in 2025, accounting for 42.94% revenue share, owing to the increased integration of AI‑driven image analysis that automates zone diameter interpretation and MIC determination, reducing subjective variability and enabling smaller veterinary labs to achieve standardized susceptibility results with minimal specialist oversight.
The disks & plates segment is estimated to register the fastest CAGR of 8.35% during the forecast period. This growth is fuelled by the adoption of chromogenic and selective agar plates designed specifically for differentiating resistant veterinary pathogens, which improves interpretive clarity and reduces retesting.
By Product Market Share (%), 2025
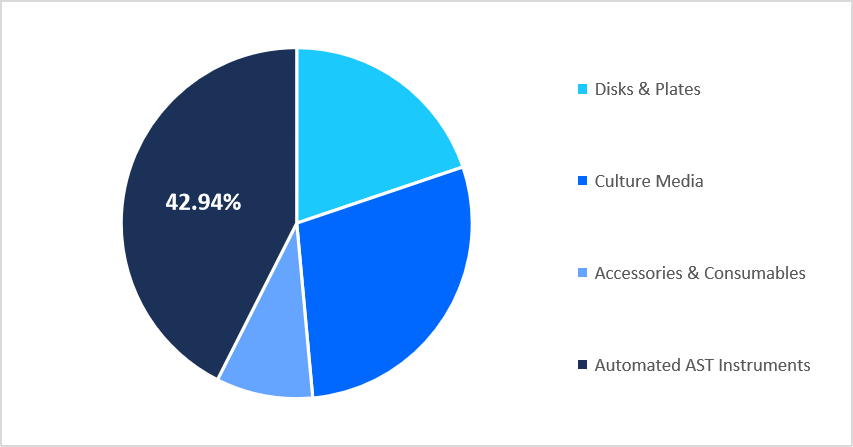
Source: Straits Research
Animal Type Insights
The production animal segment dominated the market and accounted for the highest revenue share in 2025. This growth is attributed to the mandated integration of pathogen-specific AST profiling for export certification of livestock products, where countries like Brazil require detailed antimicrobial resistance documentation for poultry and swine shipments to the EU, directly boosting routine AST adoption in commercial herds.
The companion animal segment is projected to register the fastest CAGR of 8.14% during the forecast timeframe, owing to the increasing implementation of standardized pre‑antibiotic culture and susceptibility panels for frequent canine and feline infections in small animal practices, driven by updated clinical guidelines.
End Use Insights
The veterinary reference lab segment dominated the market in 2025. This growth is driven by the development and deployment of centralized high-complexity AST panels specifically tailored to emerging zoonotic pathogens, which reference labs alone can validate and offer due to their scale and specialized expertise. These panels support multiorganism resistance profiling required for public health surveillance, attracting high sample volumes from clinics and farms.
The vet research institutes segment is expected to register a CAGR of 8.69% during the forecast period, due to the creation of curated antimicrobial resistance biomarker libraries specific to understudied veterinary pathogens, enabling advanced genotype-phenotype correlation studies and fueling demand for research-grade AST platforms and custom analytical services.
Competitive Landscape
The global veterinary antimicrobial susceptibility testing market is moderately fragmented, with major diagnostics and life science firms securing notable shares through innovation, extensive AST portfolios, and broad laboratory networks. Leading players differentiate via automated platforms, standardized consumables, and integrated data solutions while collaborating with veterinary labs and surveillance agencies. Prominent companies include bioMérieux, Thermo Fisher Scientific, IDEXX Laboratories, BD, Bruker, and others, driving clinical adoption and market expansion.
Specific Diagnostics, Inc.: An emerging market player
Specific Diagnostics, a U.S.-based biotech innovator, is gaining traction in the veterinary antimicrobial susceptibility testing market with its rapid phenotypic AST system designed to deliver susceptibility results in reduced timeframes, addressing a key veterinary demand for same-day diagnostic turnaround. Its technology, recognized with FDA Breakthrough Device designation for rapid AST performance, is being integrated into broader diagnostic offerings, enhancing veterinary laboratory efficiency and antimicrobial stewardship capabilities.
List of Key and Emerging Players in Veterinary Antimicrobial Susceptibility Testing Market
- Thermo Fisher Scientific
- bioMérieux
- BD
- Bio-Rad Laboratories Inc.
- Bruker Corporation
- Danaher
- IDEXX Laboratories
- Neogen Corporation
- HiMedia Laboratories
- Liofilchem
- Mast Group
- Hardy Diagnostics
- Condalab
- SSI Diagnostica
- Synbiosis
- Eurofins Scientific
- Merck KGaA
- QIAGEN
- Microbiologics
- Others
Strategic Initiative
- September 2025: Bioguard launched miniAST at the WSAVA Congress in Rio de Janeiro, Brazil. miniAST is a veterinary antibiotic susceptibility test analyzer to support the global fight against antimicrobial resistance.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.22 Billion |
| Market Size in 2026 | USD 1.31 Billion |
| Market Size in 2034 | USD 2.43 Billion |
| CAGR | 7.97% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product (2026-2034), By Animal Type (2026-2034), By End Use (2026-2034) |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Veterinary Antimicrobial Susceptibility Testing Market Segments
By Product (2026-2034)
- Disks & Plates
- Culture Media
- Accessories & Consumables
- Automated AST Instruments
By Animal Type (2026-2034)
- Production Animal
- Cattle
- Poultry
- Pigs
- Others
- Companion Animal
- Dogs
- Cats
- Horses
By End Use (2026-2034)
- Veterinary Reference Lab
- Vet Research Institutes
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































