Viral Vaccines Market Size, Share & Trends Analysis Report By Type (Preventive Vaccine, Therapeutic Viral), By Indication (Influenza, Human Papillomavirus, Hepatitis, Measles/Mumps/Rubella), By Route of Administration (Intramuscular and Subcutaneous Administration, Oral Administration), By Patient Type (Pediatric, Adult), By Distribution Channel (Hospitals and Retail Pharmacies, Government Suppliers) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Viral Vaccines Market Size
The global viral vaccines market size was valued at USD 55.07 billion in 2024 and is projected to grow from USD 57.72 billion in 2025 to reach USD 79.27 billion by 2033, growing at a CAGR of 4.05% during the forecast period (2025-2033).
Viral vaccines are medical preparations designed to protect against viral infections by stimulating the immune system to recognize and fight specific viruses. They typically contain weakened, inactivated, or pieces of the virus (such as proteins) to provoke an immune response without causing the disease itself. The immune system then "remembers" how to fight the virus, providing long-term immunity.
Viral vaccines are essential tools in preventing widespread viral infections and reducing severe outcomes. The major factors propelling the market growth are technological advancements, growing global health concerns, increasing need for preventative health solutions, and support from the public and private sectors.
Moreover, since the COVID-19 pandemic, worldwide collaborations between government bodies, research institutes, and pharmaceutical firms have greatly accelerated the development of viral vaccines, creating a more responsive and dynamic market.
Below graph shows the vaccination coverage of the season’s flu in Canada from 2023-2024
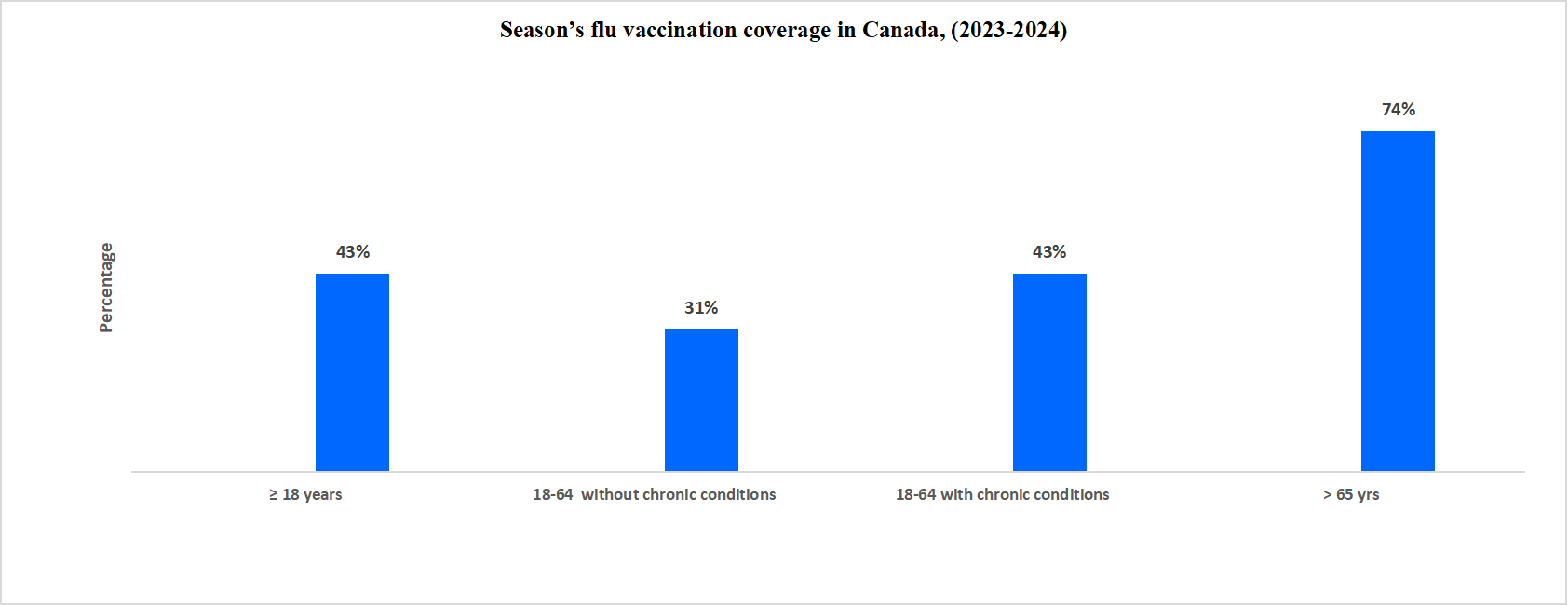
Source: Government of Canada, and Straits Research
According to the data, flu vaccination coverage was highest among individuals aged 65 and above, while it was lower among the 18-64 age group without chronic conditions. The market is seeing a growing focus on vaccination programs, driven by increased awareness of their importance in preventing harmful infections. Moreover, advancements in vaccine technology, including the use of bioreactors and the integration of AI and ML, are enhancing efficiency and treatment precision, creating new opportunities for expansion in the vaccination sector.
Viral Vaccines Market Trends
Growing Role of Bioreactors in Viral Vaccine Production
A bioreactor is an apparatus in which controlled conditions are given for the biological reaction to occur to produce cell-based viral vaccines. The emphasis on bioreactors in the production of viral vaccines is a major trend that is boosting the viral vaccines market based on their capacity to manufacture vaccines in large quantities while maintaining quality.
-
For instance, in January 2022, Univercells Technologies reported that their scale-Xbioreactor platform improves the manufacturing of viral vaccines by employing fixed-bed technology, which reduces operating volumes while producing yields that are up to 100 times greater, thereby significantly increasing productivity.
Thus, the growing role of bioreactors in viral vaccines boosts the viral vaccines market as it is particularly effective at producing clinical and commercial batches of vaccines like Lassa Fever and Rubella, as well as viral vectors like lentivirus and adenovirus.
Integration of Ai and Ml in Viral Vaccine Development
Integration of AI and ML in viral vaccines is accelerating the speed and accuracy of identifying the viral target and forecasting immune responses. To improve vaccination efficacy and enable prompt responses to newly developing viral infections, AI and ML also assist in the analysis of massive datasets to optimize vaccine formulations by cutting down on development time and costs.
-
For instance, in March 2024, a study by the University of Cincinnati and Northwestern University developedan AI model that forecasts a person's tendency to get a vaccination. By using minimal data, such as demographic information and subjective assessments like risk aversion, the algorithm can predict vaccine uptake with high accuracy.
Such innovations together propel the vaccine market's expansion by facilitating more specialized, prompt, and effective solutions.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 55.07 Billion |
| Estimated 2025 Value | USD 57.72 Billion |
| Projected 2033 Value | USD 79.27 Billion |
| CAGR (2025-2033) | 4.05% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Merck & Co., Inc., GSK plc, Sanofi, CureVac SE, Pfizer Inc. |

to learn more about this report Download Free Sample Report
Viral Vaccines Market Growth Factors
High Prevalence Rates of Viral Infectious Diseases
The global demand for viral vaccines is largely driven by the increasing prevalence and incidence of viral diseases such as COVID-19, influenza, and hepatitis, alongside the rise of new, emerging infections.
-
For example, in November 2024, the World Health Organization (WHO) reported that 10.3 million people worldwide were affected by measles. In response, accelerated immunization activities were carried out by WHO, the Measles and Rubella Partnership, and other international partners. These efforts successfully reduced the death rate through vaccination.
As the incidence of such diseases rises, the need for effective vaccines to prevent their spread and protect populations grows, thus fueling the expansion of the viral vaccines market.
Global Partnerships Driving Vaccine Production and Market Growth
Public and private sector collaborations are essential drivers of the global viral vaccines market. Pharmaceutical companies, in particular, play a pivotal role by partnering with governments and international organizations to speed up vaccine production and distribution.
- A notable example occurred in March 2024, when African nations joined forces to enhance local vaccine production through a new agreement that established a pooled procurement mechanism for vaccines and healthcare supplies.
These partnerships ensure that vaccines are more accessible, enable rapid responses to emerging diseases, and support global health initiatives. Such collaborative efforts are critical to expanding vaccine availability and strengthening health systems worldwide.
Market Restraining Factors
Adverse Reactions and Safety Concerns
Adverse reactions and safety concerns are major challenges for the viral vaccines market. To ensure widespread acceptance, it is crucial to conduct thorough safety testing and maintain transparent communication regarding potential risks. This is essential for sustaining public trust in vaccines.
-
For instance, a study published in Nature magazine in February 2024 highlighted that Arab communities across six nations reported negative side effects from the COVID-19 vaccine, including bone pain and myalgia. These severe reactions contributed to a decline in vaccine acceptance, which in turn can slow the market's overall growth.
As safety concerns continue to emerge, addressing these issues is vital for maintaining confidence in vaccination programs and ensuring continued market expansion.
Key Market Opportunities
Expansion of Mrna Vaccine Technology
The global viral vaccines market is undergoing a transformative shift with the rapid growth of mRNA vaccine technology, which is known for its speed, flexibility, and broad potential across a variety of diseases. This innovative technology is unlocking new possibilities for developing vaccines for numerous viral infections.
-
For instance, in January 2023, Moderna announced that its mRNA-1345 vaccine, aimed at protecting against Respiratory Syncytial Virus (RSV), successfully met the primary efficacy goals in a Phase 3 trial with older adults. This accomplishment highlights the promise of mRNA technology in addressing unmet medical needs.
As mRNA technology continues to advance, it is set to revolutionize the way vaccines are developed, offering faster responses to emerging viruses and ultimately improving global public health, thereby creating new growth opportunities in the viral vaccines industry.
Regional Insights
North America remains the dominant market for the global viral vaccines market, with 41.23% market share, particularly driven by the U.S. due to its advanced healthcare infrastructure, high vaccine demand, strong public healthcare initiatives, and the presence of key market players. The region’s faster-growing vaccination campaigns, such as for COVID-19 and influenza, contribute significantly to the market share.
The U.S., being the home of the largest pharmaceutical companies, is developing new vaccine technologies, including mRNA vaccines and viral vector vaccines. Moreover, the Centers for Disease Control and Prevention U.S., a public health organization, plays an essential role in spreading the importance related to vaccination programs.
- U.S. – The U.S. dominates the viral vaccines market, supported by an advanced healthcare system, cutting-edge technology in the vaccine sector, and strong public health infrastructure. Agencies like the Centers for Disease Control and Prevention (CDC) lead vaccination initiatives, while major pharmaceutical companies such as Pfizer, Moderna, and Merck drive innovation. For example, in August 2024, the FDA approved the Pfizer-BioNTech COVID-19 vaccine targeting the Omicron variant, extending eligibility to children aged 6 months to 11 years.
- Canada – In Canada, the viral vaccines industry thrives due to strong government support through public health campaigns, ensuring vaccine accessibility. The National Advisory Committee on Immunization (NACI) regularly issues recommendations, such as the introduction of seasonal influenza vaccines for individuals aged 6 months and older. These vaccines are designed to better match circulating flu strains, enhancing their effectiveness in preventing influenza across the population.
Asia-Pacific Viral Vaccines Market Trends
Asia-Pacific is experiencing rapid growth in the viral vaccines market, with major players such as China and India leading the way. This growth is fueled by significant investments in healthcare infrastructure and the expanding biotechnology sectors focused on vaccine production and distribution. Countries in the region are working to broaden vaccination coverage for diseases like polio, hepatitis, and COVID-19, emphasizing improving access and efficiency.
- China – China's viral vaccines market is bolstered by strong government involvement and a rapidly growing pharmaceutical sector focused on developing advanced vaccine technologies. In September 2024, the South China Morning Post reported the development of a new nanovaccine capable of providing protection against all COVID-19 variants and potential future mutations. This innovation underscores China’s commitment to addressing ongoing and future viral threats through scientific advancements.
- Japan - Japan's well-established healthcare system, coupled with government-sponsored immunization programs, drives the country's viral vaccines industry. There is a growing focus on developing mRNA vaccines, particularly to combat age-related viral infections. With the support of cutting-edge research and public health policies, Japan is advancing its vaccine technologies to protect its aging population and maintain its strong public health standards in the face of emerging infectious diseases.
Europe Viral Vaccines Market Trends
- Germany – Germany's viral vaccines market benefits from robust public health initiatives and excellent healthcare infrastructure. Supported by the German Vaccination Advisory Committee, the country has developed solid vaccination programs. A notable example is in December 2024, when the German Center for Infection Research (DZIF) focused on advancing a vaccine aimed at providing long-term protection against the hepatitis C virus, contributing to the goal of global hepatitis C eradication.
- France – The France market for viral vaccines is driven by a comprehensive national healthcare system that ensures access to vaccines through public health campaigns. In August 2024, the French Ministry of Health launched a nationwide campaign to raise awareness of the blue-tongue virus and epizootic hemorrhagic disease, which were spreading across the country. This initiative highlights the country’s proactive stance on managing emerging viral threats and ensuring public health safety.
Types Insights
The live attenuated vaccine segment leads the market due to its ability to trigger a strong, long-lasting immune response with fewer doses. For example, in September 2024, the Indian Council of Agricultural Research (ICAR) developed a live attenuated vaccine to protect cattle against Lumpy Skin Disease (LSD). This vaccine's effectiveness highlights the potential of live attenuated vaccines in providing efficient and durable immunity, contributing to the segment's dominance in the market.
Consumer Groups Insights
The pediatric segment is the market leader, driven by the early administration of vaccines to protect children from various infectious diseases and prevent their spread. For instance, in March 2022, over half of the world's countries included the rotavirus vaccine as part of their childhood vaccination programs to prevent severe gastroenteritis in infants and children. This widespread adoption reflects the importance of vaccinating young populations, fueling the pediatric segment's growth.
Disease Indication Insights
The influenza disease segment holds the largest share due to the widespread impact of genetic mutations in the influenza virus, which affects large populations globally. For example, in May 2024, Duke researchers published findings in Science Translational Medicine about developing a universal flu vaccine that could offer long-lasting protection against all influenza virus strains. This innovation paves the way for more effective influenza control, solidifying the dominance of this disease segment in the market.
Route of Administration Insights
The parenteral segment dominates thanks to its faster absorption, higher efficacy, and reduced risk of contamination or degradation compared to other routes of administration. As of August 12, 2024, over 13.53 billion mRNA COVID-19 vaccines had been administered globally. These vaccines, delivered primarily through the intramuscular route, demonstrated high efficacy and played a pivotal role in the success of global vaccination campaigns, underscoring the dominance of parenteral administration.
Company Market Share
Key players in the viral vaccine industry are increasingly focused on adopting a variety of strategic business approaches to solidify their position in the market. These strategies include forming strategic collaborations with other pharmaceutical companies, research institutions, and government bodies to enhance vaccine development and distribution.
Curevac Se: An Emerging Player in the Global Viral Vaccines Market
CureVac SE is a prominent German biotech company specializing in mRNA vaccine technology, focusing on developing vaccines for COVID-19 and other infectious diseases. Leveraging its cutting-edge mRNA platform, CureVac aims to improve the body's immune response to viral infections, offering a promising solution to combat emerging and existing viral threats.
List of Key and Emerging Players in Viral Vaccines Market
- Merck & Co., Inc.
- GSK plc
- Sanofi
- CureVac SE
- Pfizer Inc.
- Moderna, Inc
- Johnson & Johnson Services, Inc.
- AstraZeneca
- Dynavax Technologies
- Zydus Group
- Biological E Limited.
- Panacea Biotec Limited
- CSL
- Serum Institute of India Pvt. Ltd.
- Bharat Biotech.
Recent Developments
- August 2024 – Novavax launched a new COVID-19 vaccine adjuvanted to include the updated 2024-2025 formula, featuring a monovalent component targeting the Omicron variant JN.1 strain of SARS-CoV-2. This new formulation offers enhanced protection against the evolving virus, addressing concerns over emerging mutations.
- February 2024 – Merck & Co., Inc. advanced its efforts in preventing human papillomavirus (HPV) with the development of the Gardasil-9 vaccine, which protects against nine HPV strains responsible for the majority of HPV-related cancers and genital warts. This vaccine has been instrumental in reducing HPV infection rates and the incidence of related cancers, such as cervical, anal, and oropharyngeal cancers.
Analyst Opinion
As per our analyst, the global market is set to experience exceptional growth, fueled by advancements and strong government support for vaccine development. The success of mRNA vaccines during the COVID-19 pandemic has showcased the technology's versatility, making it a promising solution for addressing emerging viral threats like dengue, monkeypox, and new influenza strains, thereby propelling market expansion.
Governments worldwide are actively investing in vaccine research, development, and distribution, ensuring increased access and faster response times. Major market players are broadening their vaccine portfolios, venturing into areas such as HPV and malaria, which further contributes to market growth during the forecast period.
Moreover, ongoing innovation in vaccine production technologies, coupled with global collaborations between public health organizations and the private sector, will accelerate the pace of market development, enhancing availability and efficacy across diverse populations.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 55.07 Billion |
| Market Size in 2025 | USD 57.72 Billion |
| Market Size in 2033 | USD 79.27 Billion |
| CAGR | 4.05% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Types, By Consumer groups, By Disease Indication, By Route of Administration |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Singapore, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Viral Vaccines Market Segments
By Types
- Live attenuated vaccines
- Inactivated vaccines
- Sub-Unit viral vaccines
By Consumer groups
- Pediatric
- Adults
By Disease Indication
- Cancer
- Influenza
- Human Papilloma Virus
- Rota Virus
- Poliomyelitis
- Hepatitis
- Others
By Route of Administration
- Parenteral
- Oral
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































