Vaccines Market Size, Share & Industry Analysis, By Type (Monovalent Vaccines, and Multivalent Vaccines), By Route of Administration (Oral, Parenteral, and Nasal), By Disease Indication (Viral Vaccines {Hepatitis Vaccine, Influenza Vaccine, Human Papillomavirus (HPV) Vaccine, Rotavirus Vaccine (RV), Polio Vaccine, Varicella Vaccine, COVID-19 Vaccine, Measles, Mumps and Rubella (MMR) Vaccine, and Other Vaccines}, and Bacterial Vaccines {Pneumococcal Conjugate Vaccine (PCV), Meningococcal Vaccine, Diphtheria, Tetanus, and Pertussis Vaccines, and Other Vaccines}), By Age Group (Adult, and Pediatric), and Regional Forecast, 2025-2033
Vaccines Market Size
The global vaccines market size was valued at USD 67.58 billion in 2024 and is projected to grow from USD 65.83 billion in 2025 to USD 113.70 billion by 2033, exhibiting a CAGR of 7.07% during the forecast period (2025-2033).
The global vaccines market is experiencing substantial growth, driven by increasing immunization programs, government support, and advancements in vaccine technology. The rise in demand for routine and specialized vaccines, including those for HPV, pneumococcal disease, and COVID-19, has propelled market expansion. Additionally, the emergence of next-generation platforms such as mRNA, recombinant protein subunits, and viral vector vaccines is reshaping the industry. Key players, including Pfizer, GSK, Sanofi, and emerging biotech firms, are investing heavily in R&D to develop innovative and personalized vaccines.
Further, the market is also characterized by strong international collaborations and strategic investments in vaccine production capabilities. Governments and organizations such as WHO, Gavi, and CEPI are funding large-scale immunization initiatives to improve global coverage, especially in low-income regions. The below graph depicts the distribution of annual vaccine doses across different procurement channels.
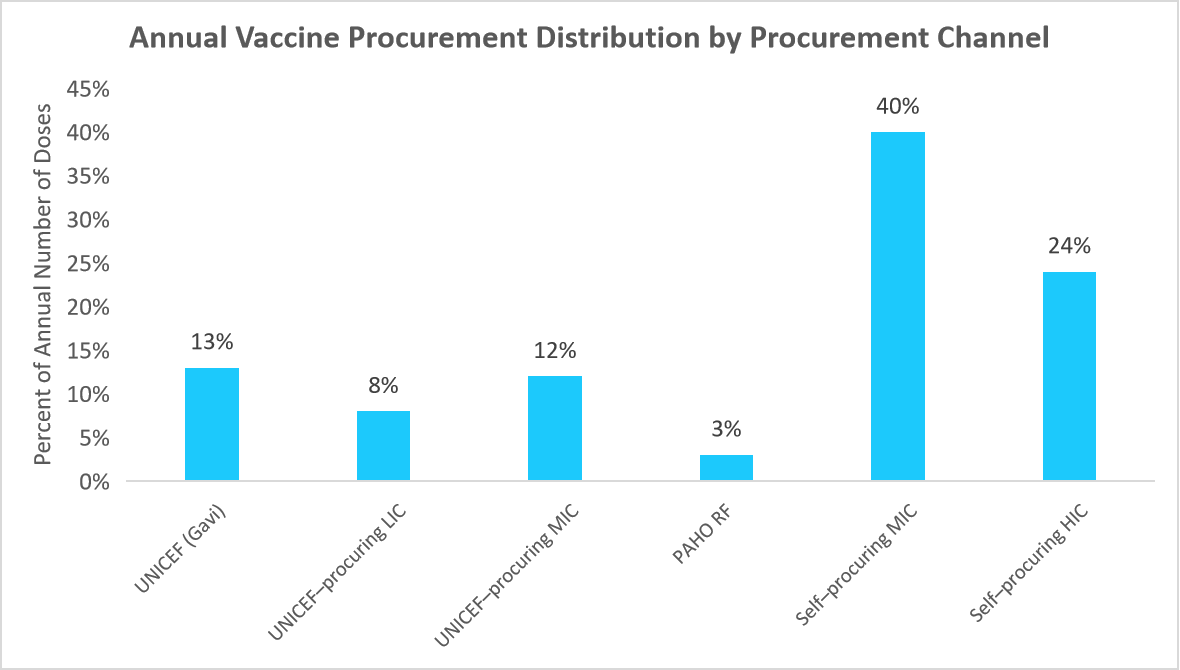
Source: United Nations Children's Fund (UNICEF), World Health Organization (WHO), and Straits Research
The global vaccine procurement landscape is diverse, with self-procuring middle-income countries (MICs) leading at 40% of annual doses, followed by self-procuring high-income countries (HICs) at 24%. UNICEF and PAHO play vital roles in supporting low- and middle-income countries, with Gavi-backed UNICEF procurement at 13% and PAHO’s Revolving Fund at 3%. While MICs are increasingly self-reliant, low-income countries (LICs) still depend on UNICEF (8%), highlighting the need for continued global funding and procurement support to ensure equitable vaccine access.
Vaccines Market Trends
Beyond Covid-19: the Mrna Vaccine Revolution
The success of mRNA-based COVID-19 vaccines has accelerated expansion beyond COVID-19, with candidates targeting flu, RSV, HIV, CMV and other emerging infectious. In addition, combination and endemic disease vaccines highlight the shift towards scalable, adaptable, and multi-target mRNA solutions.
- For example, the below table outlines the diverse pipeline of Moderna’s mRNA vaccine covering various infectious diseases beyond COVID-19.
Thus, the pipeline reflects the growing shift of vaccine developers towards mRNA technology for rapid, scalable, and adaptable vaccine development.
Moderna’s mRNA Vaccine Pipeline
| Indication | Vaccine Candidate | Development Status |
| COVID-19 vaccine | mRNA-1283 | Phase III |
| Flu vaccine | mRNA-1010 | Phase III |
| mRNA-1020 | Phase II | |
| mRNA-1030 | Phase II | |
| mRNA-1011 | Phase II | |
| mRNA-1012 | Phase II | |
| Older adults RSV vaccine | mRNA-1345 | Phase III |
| Flu + COVID vaccine | mRNA-1083 | Phase III |
| Flu + COVID + RSV vaccine | mRNA-1230 | Phase I |
| Flu + RSV vaccine | mRNA-1045 | Phase I |
| Endemic HCoV vaccine | mRNA-1287 | Preclinical Development |
| Pandemic Flu | mRNA-1018 | Phase I |
| RSV + hMPV vaccine | mRNA-1365 | Phase I |
| Pediatric RSV vaccine | mRNA-1345 | Phase II |
| CMV vaccine | mRNA-1647 | Phase III |
| EBV vaccine | mRNA-1189 | Phase I |
| mRNA-1195 | Phase I | |
| HSV vaccine | mRNA-1608 | Phase II |
| VZV vaccine | mRNA-1468 | Phase II |
| HIV vaccines | mRNA-1644 | Phase I |
| mRNA-1574 | Phase I | |
| Norovirus vaccine | mRNA-1403 | Phase I |
| mRNA-1405 | Phase II | |
| Lyme vaccine | mRNA-1975 | Phase II |
| mRNA-1982 | Phase II | |
| Zika vaccine | mRNA-1893 | Phase II |
| Nipah vaccine | mRNA-1215 | Phase I |
| Mpox vaccine | mRNA-1769 | Phase I |
Source: Company Vaccine Portfolio, Annual Report, ClinicalTrials.gov, and Straits Analysis
Digital Vaccine Management: Global Shift towards Immunization Apps
Governments across the world are adopting digital vaccine management apps to streamline immunization records, improve accessibility, and enhance public health initiatives.
- For example, the below table outlines how different countries are leveraging digital platforms to manage vaccinations and public health records.
Thus, this reflects growing reliance on technology to improve vaccine coverage, reduce administration burden, and facilitate real-time health monitoring.
Global Vaccine Management Apps and their Applications
| Country | Vaccine App | Application |
| U.S. | CDC Vaccine Schedules | Provides immunization schedules and guidelines for all age groups. |
| Canada | CANImmunize | A digital platform for tracking vaccinations and receiving reminders. |
| UK | NHS App | Allows users to access their vaccination records and health services. |
| Germany | Standing Vaccination Commission (STIKO) | Offers vaccination recommendations and schedules from Germany's health authority. |
| France | TousAntiCovid | Provides COVID-19 vaccination records, health pass, and test results. |
| India | U-WIN Vaccinator | Digital vaccine management platform for immunization tracking and scheduling. |
| Australia | Immunisation Handbook | Offers vaccination guidelines and schedules for healthcare providers and the public. |
| Brazil | Meu SUS Digital | National health app that includes vaccination records and other health services. |
Source: Health Authorities, and Straits Analysis
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 67.58 Billion |
| Estimated 2025 Value | USD 65.83 Billion |
| Projected 2033 Value | USD 113.70 Billion |
| CAGR (2025-2033) | 7.07% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Pfizer Inc., Moderna, Inc., Merck & Co., Inc., GSK plc, Sanofi |

to learn more about this report Download Free Sample Report
Vaccines Market Growth Factors
Increasing Focus on Immunization Programs
Governments and global health organizations are expanding immunization programs to improve public health and prevent disease outbreaks. Countries have established national vaccination schedules and digital immunization tracking systems to ensure widespread coverage.
- For example, the table below highlights various national immunization programs implemented worldwide.
Thus, such initiatives, coupled with digital vaccine tracking and funding support, are driving vaccines market expansion.
Global Immunization Programs by Country
| Country | Immunization Program |
| U.S. | · National Vaccine Program |
| Canada | · National Immunization Strategy |
| UK | · NHS Vaccination Schedule |
| Germany | · Vaccination Schedule |
| France | · French National Immunization Program (NIP) |
| Italy | · Italian Immunization Schedule |
| Spain | · Immunisation schedule of the Spanish Association of Pediatrics |
| Belgium | · Immunization Schedule |
| Denmark | · Childhood Vaccination Programme |
| Sweden | · National vaccination programme for children |
| Ireland | · National Immunisation Office |
| Switzerland | · Swiss Immunization Schedule |
| China | · National Immunization Program |
| India | · Universal Immunization Programme (UIP)· Mission Indradhanush |
| Australia | · National Immunisation Program Schedule |
| South Korea | · National Immunization Program |
| South Africa | · Expanded Program on Immunisation |
| Singapore | · National Childhood Immunisation Schedule (NCIS)· Vaccination and Childhood Development Screening Subsidies (VCDSS)· Private Vaccination Programme (PVP) |
| Brazil | · National Immunization Program |
| Argentina | · National Immunization Schedule |
Source: Health Authorities, Immunization Programs, and Straits Analysis
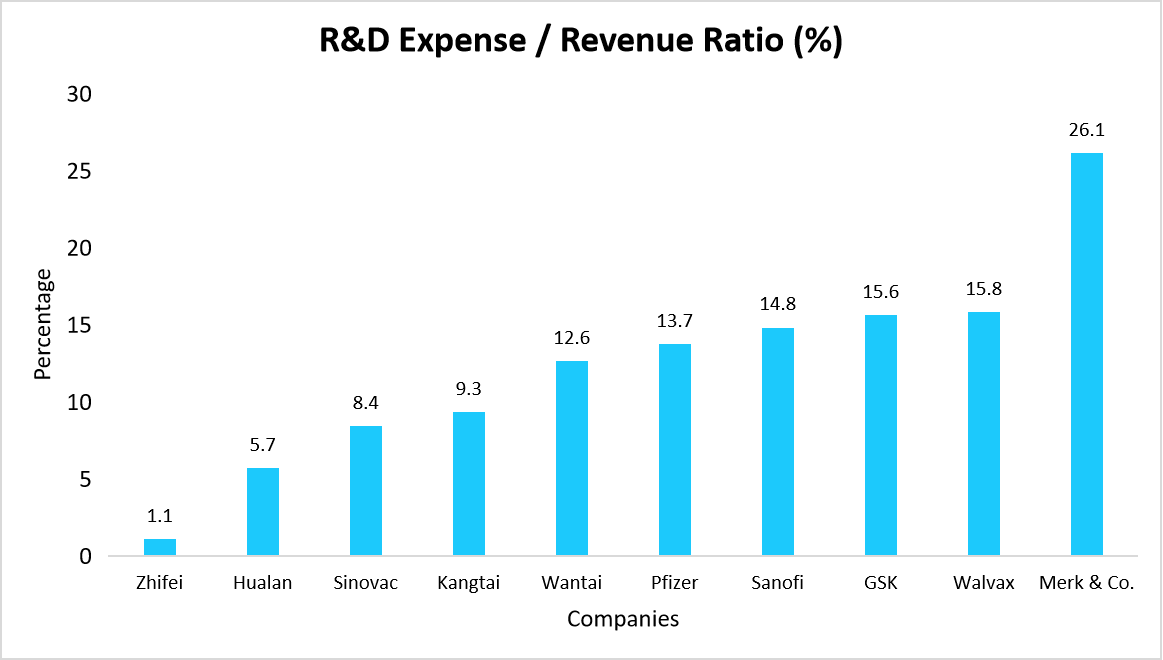
R&d Investments Propelling Vaccine Market Expansion
Pharmaceutical companies and biotech firms are increasing R&D investments to develop next-generation vaccines, improve efficacy, and expand indications. Advancements in mRNA technology, personalized cancer vaccines, and universal flu vaccines demonstrate the industry's shift toward innovation.
- For instance, Merck & Co. leads in R&D Expense to Revenue Ratio (26.1%), followed by Walvax (15.8%), GSK (15.6%), and Sanofi (14.8%), highlighting strong reinvestment in vaccine innovation.
Thus, higher R&D investment is driving vaccine innovation and market expansion.
Market Restraining Factors
Vaccine Stockouts & Supply Chain Disruptions
Frequent vaccine stockouts owing to procurement delays, funding gaps, distribution issues, and inaccurate demand forecasting pose a major challenge to immunization programs, limiting the growth of market.
- For example, the graph highlights key factors contributing to vaccine stockouts limiting the market expansion.
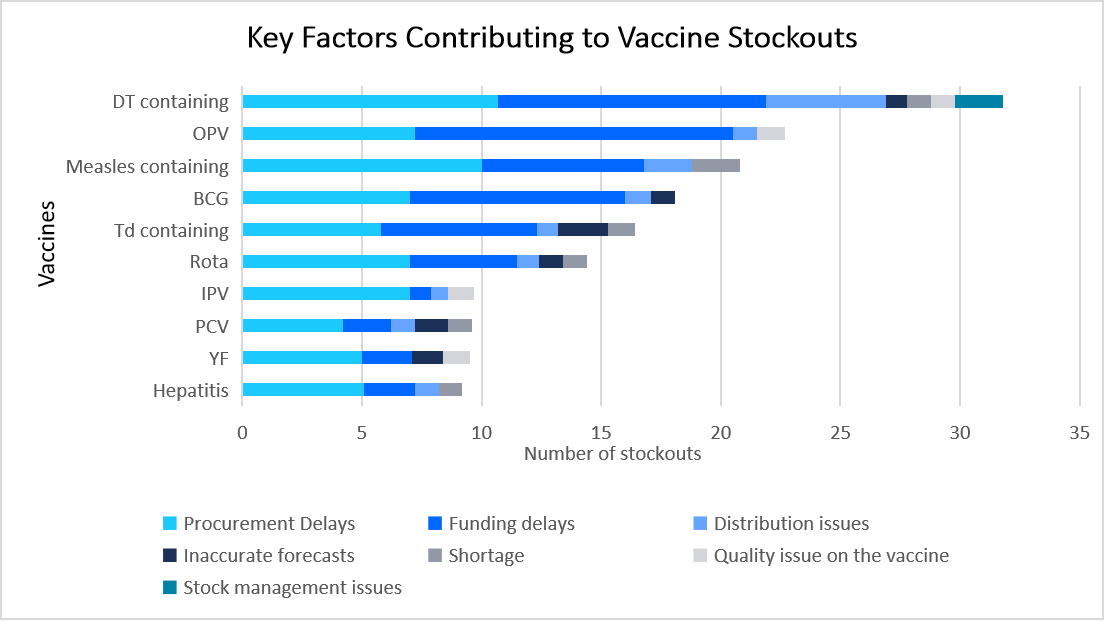
Source: United Nations Children's Fund (UNICEF), World Health Organization (WHO), and Straits Research
Market Opportunity
Growing Demand for Adult & Travel Vaccines
Rising awareness, aging populations, and government recommendations are driving demand for adult immunization and travel vaccines. Increased global mobility and emerging infectious diseases further support market expansion.
- For example, the U.S. vaccine recommendation chart highlights the need for annual influenza vaccination and updated COVID-19 doses across all adult age groups, emphasizing the growing focus on adult immunization.
U.S. Vaccine Recommendation Chart
| Vaccine | 19-26 Years | 27-49 Years | 50-64 Years | ≥65 Years |
| COVID-19 | 1 or more doses of 2024-2025 vaccine | 2 or more doses of 2024-2025 vaccine | ||
| Influenza | 1 dose annually | 1 dose annually | 1 dose annually | Annually (HD-IV3, RIV3, or allV3 preferred) |
| Respiratory Syncytial Virus (RSV) | Seasonal administration during pregnancy | 60 through 74 years (See Notes) | ≥75 years (See Notes) | |
| Tetanus, Diphtheria, Pertussis (Tdap or Td) | 1 dose Tdap, then Td/Tdap booster every 10 years | |||
| Measles, Mumps, Rubella (MMR) | 1 or 2 doses depending on indication (if born in 1957 or later) | For healthcare personnel | ||
| Varicella (VAR) | 2 doses (if born in 1980 or later) | 2 doses | ||
| Zoster Recombinant (RZV) | 2 doses for immunocompromising conditions | 2 doses | ||
| Human Papillomavirus (HPV) | 2 or 3 doses depending on age at initial vaccination or condition | 27 through 45 years | - | - |
| Hepatitis A (HepA) | 2, 3, or 4 doses depending on vaccines | |||
| Hepatitis B (HepB) | 2, 3, or 4 doses depending on vaccine or condition | |||
| Meningococcal A, C, W, Y (MenACWY) | 1 or 2 doses depending on indication | |||
| Meningococcal B (MenB) | 19 through 23 years | 2 or 3 doses depending on vaccine and indication | ||
| Haemophilus Influenzae Type b (Hib) | 1 or 3 doses depending on indication | |||
| Mpox (Monkeypox) | 2 doses | |||
| Inactivated Poliovirus (IPV) | Complete 3-dose series if incompletely vaccinated. Self-report of previous doses acceptable | |||
Source: Centers for Disease Control and Prevention (CDC), and Straits Analysis
Regional Insights
North America holds a leading position in the vaccines market owing to the presence of major players such as Pfizer, Inc., Moderna, Merck & Co., Inc., and Johnson & Johnson, which drive innovation and production. Strong government immunization programs, high healthcare expenditure, and rapid regulatory approvals further support market growth. Additionally, widespread public awareness campaigns and increasing adoption of novel vaccines contribute to the region’s dominance.
U.s. Vaccines Market Trends
- The U.S. vaccine market is experiencing strong growth, driven by increasing demand and the presence of leading domestic and international manufacturers such as Merck, Pfizer, Moderna, and Bavarian Nordic Inc. Companies such as Merck play a key role with successful products such as Gardasil (HPV vaccine), while a supportive regulatory environment, including the FDA’s stringent safety standards and the National Vaccine Injury Compensation Program (VICP), encourages continued investment in vaccine R&D.
Asia Pacific Vaccines Market Trends
The Asia-Pacific region is expected to register the fastest CAGR during the forecast period owing to rising government initiatives for immunization, increasing healthcare investments, and growing awareness of vaccine-preventable diseases. Expanding biotechnology and pharmaceutical industries, particularly in China and India, further support market growth.
- China’s vaccine market is one of the largest globally, driven by strong government support, rising immunization demand, and advancements in next-generation vaccine manufacturing. The market is divided into state-funded Expanded Program on Immunization (EPI) vaccines and privately purchased non-EPI vaccines, with high-value products such as HPV and pneumococcal vaccines gaining traction. Key players such as Sinopharm CNBG, Sinovac, CanSino, and AIM Vaccine dominate both the segments. Additionally, China’s "vaccine diplomacy" has strengthened its global influence, particularly in developing regions. The below graph shows the increasing non-EPI vaccines adoption owing to increasing demand of COVID-19, HPV and PCV13 vaccines.
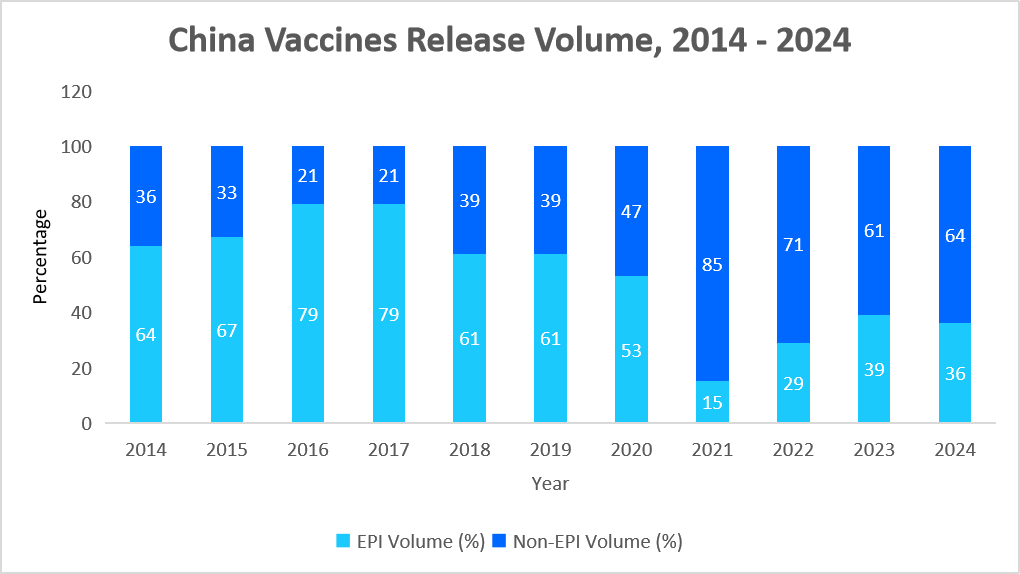
Source: Clinton Health Access Initiative (CHAI) – China and Straits Research
- The Indian market is a rapidly growing segment in the global pharmaceutical industry, supplying over 60% of the world's vaccines, particularly to LMICs. Driven by strong manufacturing capabilities, cost-effective production, and increasing global demand, key players such as Serum Institute of India, Bharat Biotech, and Biological E dominate the market. India exports vaccines for polio, measles, and diphtheria, with reports indicating it supplies 65-65-70% of global measles vaccine demand. The below graph highlights India's expanding vaccine exports, with a growing share directed toward lower-middle-income countries
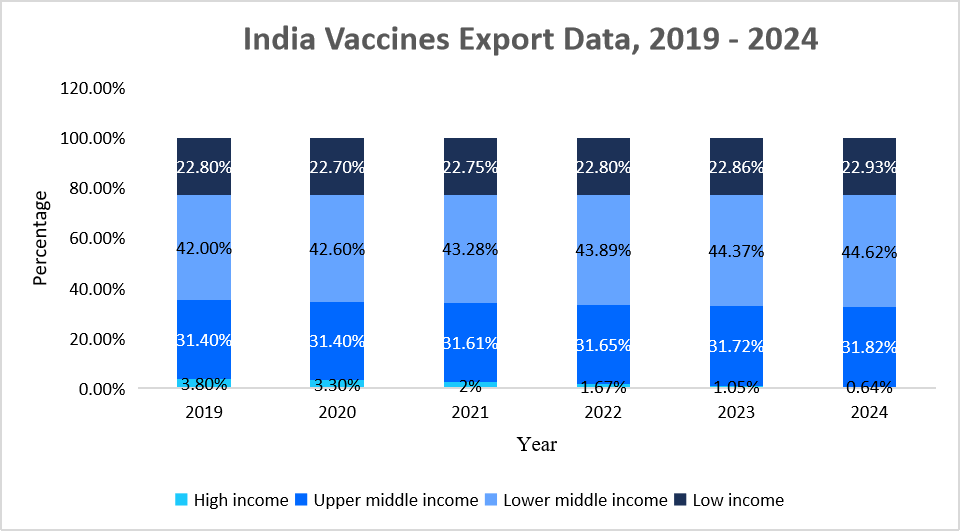
Source: World Integrated Trade Solution (WITS), and Straits Research
Europe Market Trends
- Germany’s vaccine market is growing steadily, driven by government policies aimed at improving adult vaccination uptake. Initiatives such as “Influencing Policy to Improve Adult Vaccination in Germany” have engaged healthcare professionals to boost immunization rates. Vaccine willingness has risen in recent years, supported by high public trust in childhood vaccinations, driving the demand for vaccines.
- France’s market is expanding owing to strong government support and extensive immunization programs promoting vaccination awareness. Collaborative research efforts by major pharmaceutical companies further drive growth, such as the August 2024 partnership between Valneva and LimmaTech to develop a tetravalent Shigella bioconjugate vaccine. Strategic initiatives such as the Vaccine Research Institute’s plan and France’s Global Health Strategy further enhance vaccine adoption and market expansion.
- Belgium’s market thrives on a strong healthcare infrastructure, a skilled workforce, and the presence of major vaccine producers such as GSK and Pfizer, reinforcing its leadership in vaccine development and innovation. Additionally, Belgium’s government actively supports public health initiatives, including funding for Gavi and the Be4OneW project, which enhances biopharmaceutical production and addresses global vaccination challenges.
- The UK market is expanding owing to joint government-private initiatives for personalized vaccines, increasing clinical trials for mRNA cancer vaccines, and strategic investments in next-generation vaccine development. The UK aims to enhance domestic vaccine production, with facilities such as Vaccines Manufacturing & Innovation Center (VMIC) bolstering national preparedness. Collaborations with WHO and Gavi, along with significant funding through the International Finance Facility for Immunization (IFFI), support global vaccine accessibility. The below graph shows UK’s contribution to IFFI for vaccination coverage.
_2.png)
Source: GAVI-UK, and Straits Research
Type Analysis
The global market is bifurcated into monovalent vaccines and multivalent vaccines. Multivalent vaccines segment is expected to register the fastest CAGR owing to its ability to provide immunity against multiple pathogens in a single dose, reducing the number of injections required, improving patient compliance, and enhancing immunization coverage.
- For instance, CureVac announced interim Phase 2 data from its ongoing Phase 1/2 study of its seasonal influenza multivalent mRNA vaccine candidate, developed in collaboration with GSK plc.
Route of Administration Analysis
The global market is oral, parenteral, and nasal. Parenteral segment accounted for the largest share driven by the widespread use of injectable vaccines, higher efficacy, and well-established immunization programs.
- For instance, in February 2025, Bavarian Nordic announced FDA approval of VIMKUNYA™, the first single-dose virus-like particle (VLP) chikungunya injection vaccine in the U.S. for individuals aged 12 and older.
Disease Indication Analysis
The market is categorized into viral vaccines and bacterial vaccines. Viral vaccines dominate the market owing to their widespread use in immunization programs against prevalent infectious diseases such as influenza, COVID-19, hepatitis, MMR, and HPV. The high burden of viral infections, continuous advancements in vaccine technology, and strong government support for large-scale immunization campaigns further drives their market dominance.
- For instance, in December 2024, Bavarian Nordic has signed a license and manufacturing agreement with Serum Institute of India (SII) for its MVA-BN viral mpox vaccine.
Age Group Analysis
The market is categorized into adult and pediatric. Adult segment is expected to register the fastest CAGR owing to the rising awareness of adult immunization, increasing prevalence of infectious diseases among adults, and growing government recommendations for booster doses and travel vaccines.
- For instance, Merck & Co., Inc., announced that China's NMPA has approved GARDASIL (HPV Quadrivalent Vaccine) for males aged 9-26 to prevent certain HPV-related cancers and diseases.
Company Market Share
Key players in the industry are focus on adopting key business strategies, such as strategic collaborations, product approvals, acquisitions, and product launches, to gain a strong foothold in the market.
Bavarian Nordic Inc.: An Emerging Player in the Vaccines Market
Bavarian Nordic Inc. is an emerging player in the vaccine market, specializing in viral vector-based vaccines for infectious diseases such as smallpox, monkeypox, and rabies.
Recent developments by Bavarian Nordic Inc.:
- In March 2025,Bavarian Nordic A/S announced that the UK MHRA has validated its marketing application and begun reviewing its single-dose CHIKV VLP vaccine for chikungunya prevention in individuals aged 12 and older.
List of Key and Emerging Players in Vaccines Market
- Pfizer Inc.
- Moderna, Inc.
- Merck & Co., Inc.
- GSK plc
- Sanofi
- BioNTech SE.
- Johnson & Johnson
- China National Biotec Group Company Limited (CNBG)
- AstraZeneca
- Serum Institute of India (SII)
- Bejing
- CSL Behring
- Walvax Biotechnology Co., Ltd.
- Bavarian Nordic Inc.
- Takeda Pharmaceutical Company Limited
Recent Developments
- In February 2025, GSK announced FDA approval for Penmenvy, a meningococcal (A, B, C, W, Y) vaccine for individuals aged 10-25 years.
- In January 2024, Indian Immunologicals Ltd. (IIL), a biopharmaceutical business in India fully owned by the National Dairy Development Board (NDDB), introduced 'Havisure,’ India's first Hepatitis A vaccine developed within the country.
Analyst Opinion
The vaccines market is witnessing significant growth, driven by increasing demand for immunization, advancements in next-generation vaccine technologies, and strong government support worldwide. Key players such as Merck, Pfizer, GSK, and emerging biotech firms are accelerating R&D efforts, particularly in mRNA, recombinant protein, and personalized vaccines. Regulatory agencies are streamlining approval processes, fostering innovation while ensuring safety and efficacy. Additionally, global vaccination initiatives and funding from organizations such as Gavi and WHO are expanding access in emerging markets. Moreover, as new infectious threats arise, continued investments in vaccine development and manufacturing capacity will be crucial for sustained market expansion.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 67.58 Billion |
| Market Size in 2025 | USD 65.83 Billion |
| Market Size in 2033 | USD 113.70 Billion |
| CAGR | 7.07% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Route of Administration, By Disease Indication, By Age Group |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Singapore, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Vaccines Market Segments
By Type
- Monovalent
- Multivalent
By Route of Administration
- Oral
- Parenteral
- Nasal
By Disease Indication
-
Viral
- Hepatitis Vaccine
- Influenza Vaccine
- Human Papillomavirus (HPV) Vaccine
- Rotavirus Vaccine (RV)
- Polio Vaccine
- Varicella Vaccine
- COVID-19 Vaccine
- Measles, Mumps and Rubella (MMR) Vaccine
- Other Vaccines
-
Bacterial
- Pneumococcal Conjugate Vaccine (PCV)
- Meningococcal Vaccine
- Diphtheria, Tetanus, and Pertussis Vaccines
- Other Vaccines
By Age Group
- Pediatric
- Adult
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Mitiksha Koul
Research Associate
Mitiksha Koul is a Research Associate with 2 years of experience in market research. She focuses on analyzing industry trends, competitive landscapes, and growth opportunities to support strategic decision-making. Mitiksha’s strong analytical skills and research expertise enable her to deliver actionable insights that help businesses adapt to evolving market dynamics and achieve sustainable growth.










































