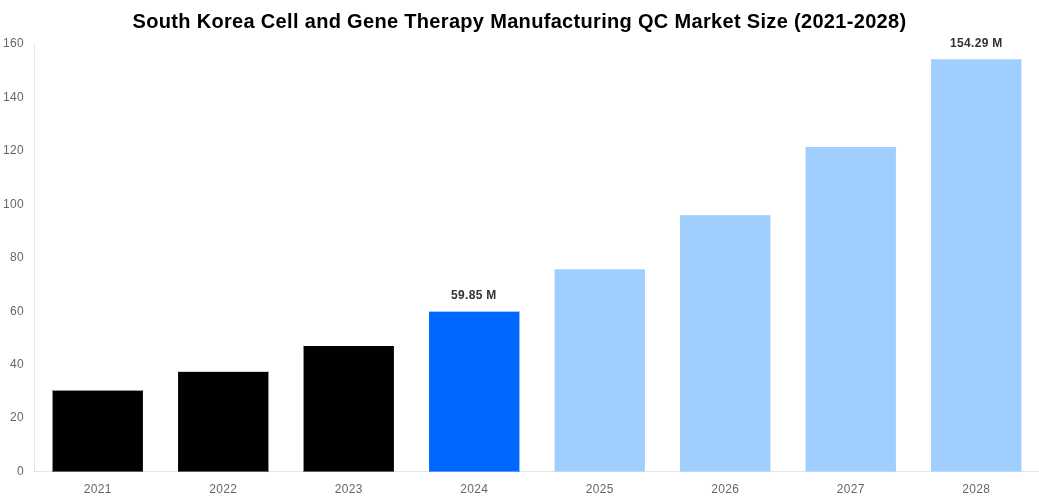
South Korea Cell and Gene Therapy Manufacturing QC Market Size
According to Straits Research analysis, the South Korea Cell and Gene Therapy Manufacturing QC Market was valued at USD 59.85 Million in 2024 and is projected to reach USD 154.29 Million by 2028, expanding at a CAGR of 26.8% during the forecast period. The market growth is primarily driven by an increasing demand from the healthcare industry, especially in the fields of personalized medicine and regenerative therapies. It is further bolstered by progressive applications in agriculture and energy sectors. The chemical industry is also a significant driver, as this technology is extensively used for product quality assurance. Towards sustainability, there is a trend to optimize these therapies for lower carbon production. Technological advancements are continually opening new opportunities for this market. As exports continue to soar, South Korea is poised to become a global hub for Cell and Gene Therapy Manufacturing QC, marking a critical milestone in the global marketplace.
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market Highlights
- Cell Therapy held the leading position among Therapy Type segments in 2024, based on market size.
- Gene Therapy is projected to post the fastest growth rate, sustaining its position as the most attractive Therapy Type segment during the forecast horizon.
- South Korea accounted for 2.68% of the global cell and gene therapy manufacturing qc market size in 2024.
- By 2028, United States is expected to remain the top global market in terms of size.
- Within Asia Pacific, China is forecasted to dominate the regional cell and gene therapy manufacturing qc market size by 2028.
- India will be the fastest-growing market in Asia Pacific, projected to achieve USD 58503 Million by 2028.
- 📊 Preview Report Scope and Structure – Gain immediate visibility into key topics, market segments, and data frameworks covered.
- 📥 Evaluate Strategic Insights – Access selected charts, statistics, and analyst-driven commentary derived from the final report deliverables.
Report Summary
| Report Scope |
Details |
| Base Year for Study |
2024 |
| Study Period |
2021-2028 |
| Historical Period |
2021-2023 |
| Forecast Period |
2025-2028 |
| Market Size In 2024 |
USD 59.85 Million |
| Market Size In 2028 |
USD 154.29 Million |
| Largest segment |
Cell Therapy |
| Fastest segment |
Gene Therapy |
| Units |
Revenue in USD Million |
| CAGR |
26.8% (2025-2028) |
| Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
South Korea Cell and Gene Therapy Manufacturing QC Market Share By Therapy Type
Segmentation Covered
| Segments |
Sub Segments |
| Therapy Type |
- Cell Therapy
- Gene Therapy
|
| Cell Therapy |
- CAR-T
- CAR-NK
- B-Cell
- Other
|
| CAR-T |
- Autologous CAR-T
- Allogeneic CAR-T
|
| Gene Therapy |
- Viral
- Non-Viral
|
| Viral |
- AAV
- Lentiviral vectors
- Other
|
| Scale |
- Pre commercial/ R&D Manufacturing
- Commercial Scale Manufacturing
|
| Mode |
- Contract Manufacturing
- In-house Manufacturing
|
| Workflow |
- Cell Processing
- Cell Banking
- Fill & Finish Operations
- Raw Material Testing
- Vector Production
- Other Workflows
|
| Process |
- Upstream Processes
- Downstream Processes
|
| End User |
- Pharmaceutical Companies
- Biopharmaceutical / Biotechnological Companies
- Contract Manufacturing Organizations
|
| Pharmaceutical Companies |
- Small Size
- Medium Size
- Large Size
|
| Biopharmaceutical / Biotechnological Companies |
- Small Size
- Medium Size
- Large Size
|
| Contract Manufacturing Organizations |
- Small Size
- Medium Size
- Large Size
|
| Technology Type |
- Fluorescence-Activated Cell Sorting (FACS)
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Chromatography
- Next-Generation Sequencing (NGS)
- Polymerase Chain Reaction (PCR)
- Others
|
South Korea Cell and Gene Therapy Manufacturing QC Market By Therapy Type 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Cell Therapy 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By CAR-T 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Gene Therapy 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Viral 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Scale 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Mode 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Workflow 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Process 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By End User 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Pharmaceutical Companies 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Biopharmaceutical / Biotechnological Companies 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Contract Manufacturing Organizations 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.
South Korea Cell and Gene Therapy Manufacturing QC Market By Technology Type 2028
Source: Straits Research Analysis Company Publications, Primary Interviews.