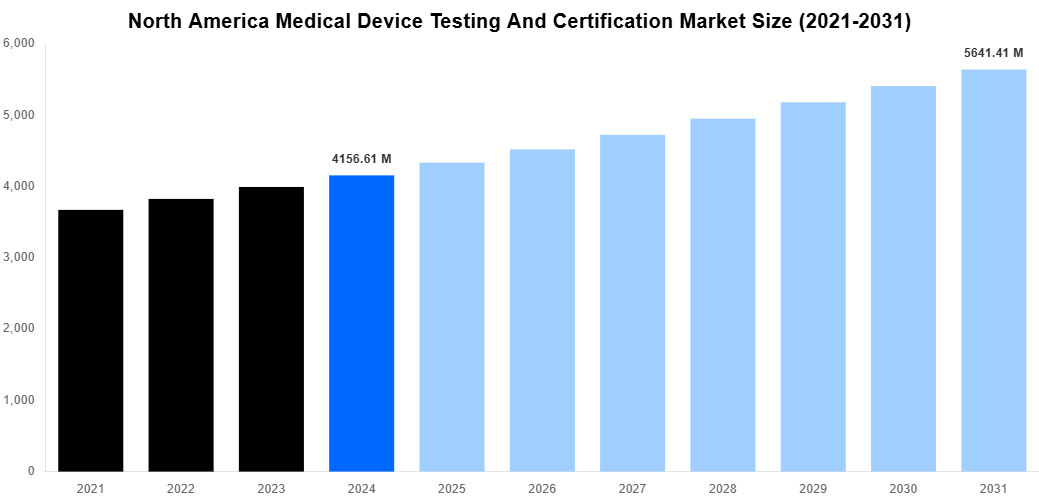
North America Medical Device Testing And Certification Market Size
According to Straits Research analysis, The North America Medical Device Testing And Certification Market was valued at USD 4156.61 Million in 2024 and is projected to reach USD 5641.41 Million by 2031, expanding at a CAGR of 4.4% during the forecast period. The primary industries propelling this growth are the healthcare and life sciences sector, leveraging advanced medical devices for improved patient outcomes. Regulatory mandates by authorities like the FDA necessitate rigorous testing and certification, bolstering market demand. Increasing emphasis on green production, sustainability, and technological advancements — such as AI and IoT in medical devices — contribute to emerging trends of this sector. Export potential remains untapped, especially in innovative diagnostic devices. Going forward, the North American region is strategically positioning itself to induce growth while adhering to regulatory and sustainability requirements in medical device testing and certification.
Source: Straits Research Analysis Company Publications, Primary Interviews.
North America Medical Device Testing And Certification Market Highlights
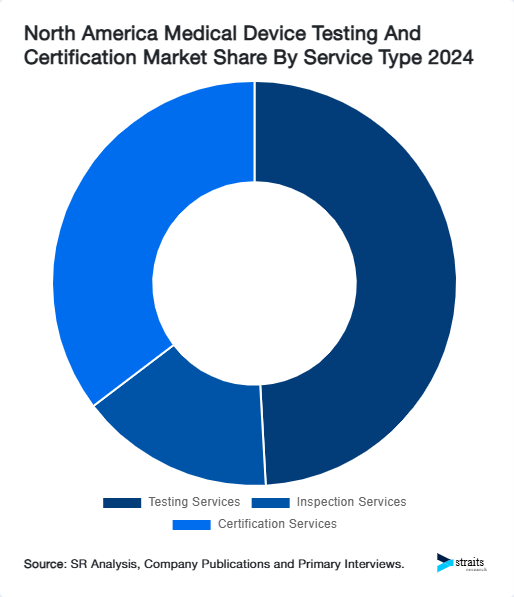
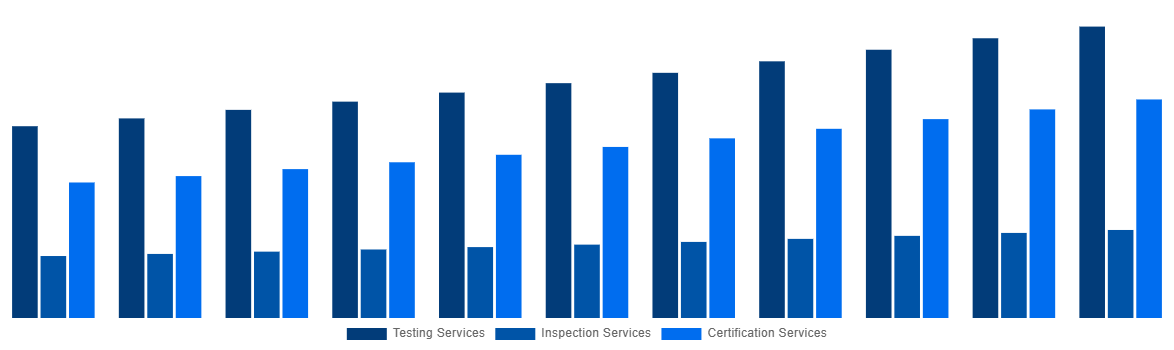
- By market size, Testing Services led the Service Type category in 2024.
- The Service Type segment led by Certification Services is estimated to post the fastest growth, sustaining its position as the most lucrative during the forecast timeframe.
- In 2024, North America contributed 41.28% to the overall global medical device testing and certification market size.
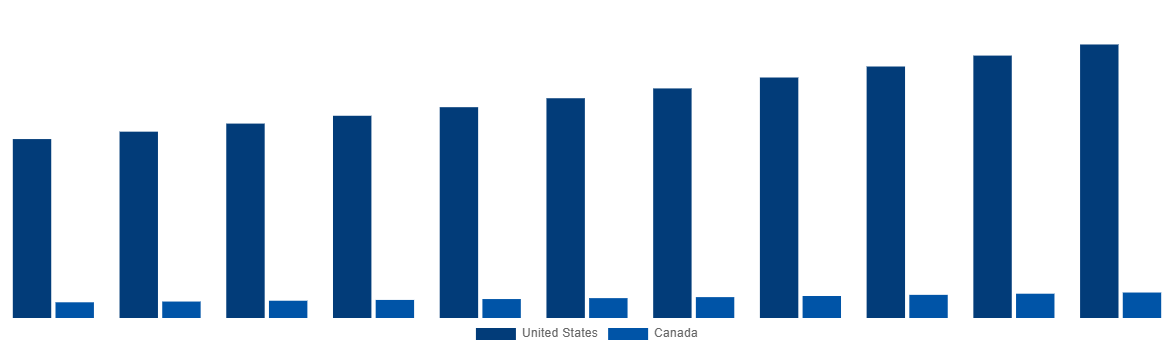
- By 2031, United States is anticipated to dominate the global market based on market size.
- Canada is expected to witness the fastest growth within North America, attaining USD 735.73 Million by 2031.
- 📊 Preview Report Scope and Structure – Gain immediate visibility into key topics, market segments, and data frameworks covered.
- 📥 Evaluate Strategic Insights – Access selected charts, statistics, and analyst-driven commentary derived from the final report deliverables.
Report Summary
| Report Scope |
Details |
| Base Year for Study |
2024 |
| Study Period |
2021-2031 |
| Historical Period |
2021-2023 |
| Forecast Period |
2025-2031 |
| Market Size In 2024 |
USD 4156.61 Million |
| Market Size In 2031 |
USD 5641.41 Million |
| Largest segment |
Testing Services |
| Fastest segment |
Certification Services |
| Units |
Revenue in USD Million |
| CAGR |
4.4% (2025-2031) |
| Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
North America Medical Device Testing And Certification Market Share By Service Type
Segmentation Covered
| Segments |
Sub Segments |
| Country |
- United States
- Canada
|
| Service Type |
- Testing Services
- Inspection Services
- Certification Services
|
| Sourcing Type |
- In-house
- Outsourced
|
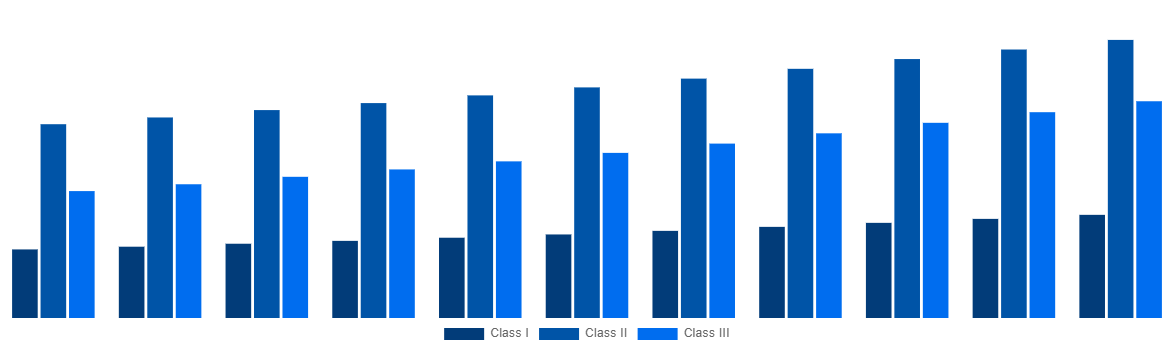
| Device Class |
- Class I
- Class II
- Class III
|
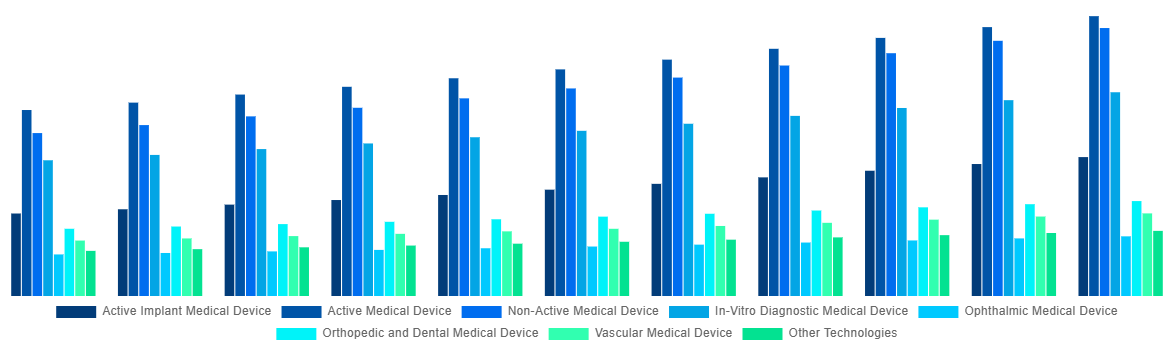
| Technology |
- Active Implant Medical Device
- Active Medical Device
- Non-Active Medical Device
- In-Vitro Diagnostic Medical Device
- Ophthalmic Medical Device
- Orthopedic and Dental Medical Device
- Vascular Medical Device
- Other Technologies
|
North America Medical Device Testing And Certification Market By Country 2031
Source: Straits Research Analysis Company Publications, Primary Interviews.
North America Medical Device Testing And Certification Market By Service Type 2031
Source: Straits Research Analysis Company Publications, Primary Interviews.
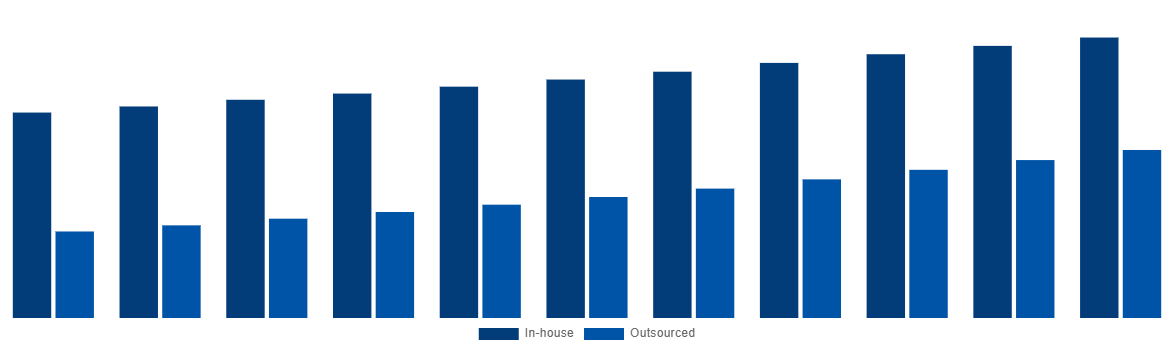
North America Medical Device Testing And Certification Market By Sourcing Type 2031
Source: Straits Research Analysis Company Publications, Primary Interviews.
North America Medical Device Testing And Certification Market By Device Class 2031
Source: Straits Research Analysis Company Publications, Primary Interviews.
North America Medical Device Testing And Certification Market By Technology 2031
Source: Straits Research Analysis Company Publications, Primary Interviews.