Antithrombin Market Size, Share & Trends Analysis Report By Source (Human, Recombinant Goat Milk), By Dosage Form (Lyophilized Powder, Liquid Form), By Application (Therapeutics, Research, Diagnostics, Others), By End User (Hospitals, Specialty Clinics, Academic & Research Institutes, Diagnostic Laboratories, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Antithrombin Market Overview
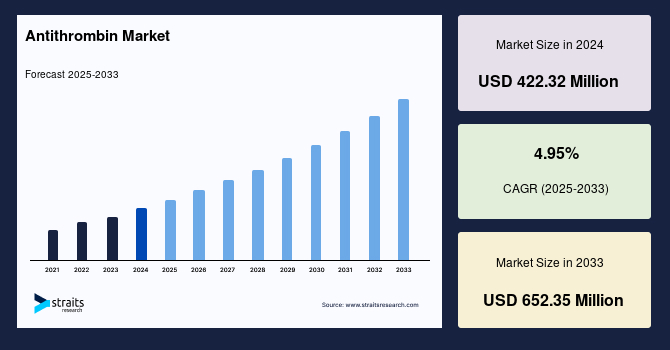
The global antithrombin market size was valued at USD 422.32 million in 2024 and is projected to grow from USD 443.23 million in 2025 to reach USD 652.35 million by 2033, growing at a CAGR of 4.95% during the forecast period (2025–2033). The growth of the market is attributed to rising incidence of thrombotic disorders.
Key Market Insights
- North America leads with strong healthcare infrastructure and rising surgical volumes, especially in organ and cardiovascular procedures.
- The U.S. market sees high adoption of recombinant antithrombin, driven by FDA approvals, R&D, and rare disease focus.
- Asia-Pacific is growing fastest due to increased thrombosis awareness, rising surgical cases, and expanding biopharma presence.
- Europe benefits from structured healthcare and has high therapeutic demand, especially in geriatric and perioperative care.
- Recombinant goat milk is emerging as a scalable, pathogen-free source of antithrombin with commercial adoption by firms like rEVO Biologics.
- Hospitals account for the largest end-user segment due to intensive use in surgeries, ICU care, and thrombotic event management.
Market Size & Forecast
- 2024 Market Size: USD 422.32 Million
- 2033 Projected Market Size: USD 652.35 Million
- CAGR (2025-2033): 4.95%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing market
A key driver of the global antithrombin market is the growing awareness and improved diagnosis of its deficiency, particularly through advanced genetic testing and coagulation screening. Early detection has increased the clinical use of this molecule, especially in high-risk settings like neonatal care and during complex surgeries. Its critical role in preventing thrombosis during procedures such as cardiopulmonary bypass and organ transplants is further fueling demand.
Another significant factor is the regulatory encouragement for developing rare disease therapies. Authorities like the U.S. FDA and EMA offer incentives, including orphan drug designation, tax credits, and market exclusivity, motivating companies to invest in antithrombin research. These combined factors, better diagnostics, rising clinical application in surgeries, and favorable regulatory frameworks, are significantly contributing to market growth, particularly in developed regions and increasingly in emerging healthcare systems across Asia-Pacific and Latin America.
Market Trend
Expanded Clinical Applications
The global market is increasingly shaped by its broadened clinical utility beyond congenital deficiencies. Antithrombin is now being explored for use in a range of conditions such as sepsis, preeclampsia, extracorporeal membrane oxygenation (ECMO), and major surgeries, especially cardiac procedures. These expanded applications are gaining traction due to the protein's dual anti-inflammatory and anticoagulant properties, making it highly relevant in complex medical settings.
- For instance, an ongoing Phase III randomized, placebo-controlled trial (ATN108) is testing Octapharma’s plasma-derived antithrombin (Atenativ) in adult cardiac surgery patients resistant to heparin. The aim is to restore heparin responsiveness during cardiopulmonary bypass. Results are anticipated in 2026, potentially offering a new standard for managing anticoagulation in this high-risk group.
Such advancements signal growing R&D investment and clinical confidence in this molecule’s evolving therapeutic value.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 422.32 Million |
| Estimated 2025 Value | USD 443.23 Million |
| Projected 2033 Value | USD 652.35 Million |
| CAGR (2025-2033) | 4.95% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
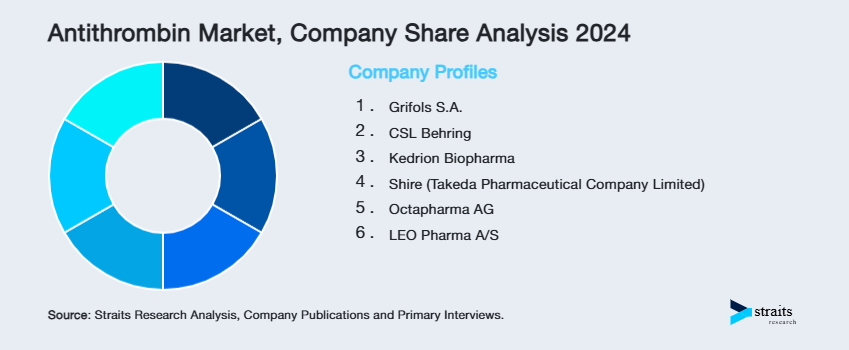
| Key Market Players | Grifols S.A., CSL Behring, Kedrion Biopharma, Shire (Takeda Pharmaceutical Company Limited), Octapharma AG |
to learn more about this report Download Free Sample Report
Market Drivers
Rising Incidence of Thrombotic Disorders
The increasing global burden of thrombotic disorders is a significant driver for the global market. Factors such as sedentary lifestyles, aging populations, obesity, and cancer contribute to the rising prevalence of conditions like venous thromboembolism (VTE), deep vein thrombosis (DVT) and pulmonary embolism (PE).
- Globally, in the eight major countries like the U.S., Germany, France, Italy, Spain, the U.K., Canada, and Japan, diagnosed venous thromboembolism (VTE) cases are projected to rise from 1.12 million in 2015 to 1.35 million by 2025, with an annual growth of ~2.1%. Of these, diagnosed deep vein thrombosis (DVT) cases are expected to climb from 801,472 in 2015 to 977,234 by 2025, growing at ~2.2% annually.
This steady surge highlights the growing demand for anticoagulant therapies, including antithrombin. Additionally, the increasing use of this molecule in surgical procedures and intensive care settings further boosts its clinical relevance and market potential.
Market Restraint
High Cost and Limited Accessibility
One of the key restraints in the global market is the high cost and limited accessibility of antithrombin therapies, particularly recombinant forms. These therapies involve complex biotechnological processes and strict regulatory requirements, resulting in elevated production and distribution costs. Consequently, prices remain high, limiting adoption in low- and middle-income countries.
In many regions, patients may lack adequate insurance coverage or government healthcare support to afford such treatments. Additionally, the limited availability of advanced diagnostic facilities and therapeutic infrastructure in developing countries further hinders widespread use. These barriers contribute to underdiagnosis and undertreatment, despite the growing prevalence of thrombotic disorders worldwide.
Market Opportunity
Strategic Partnerships and Acquisitions
Strategic partnerships and acquisitions are unlocking new growth avenues in the global market. Pharmaceutical companies are increasingly joining forces to accelerate research, improve supply chains, and expand product pipelines. These collaborations are especially crucial in rare and complex conditions where the development cost is high and time-consuming.
- For instance, in February 2022, Grifols and Endpoint Health entered a global collaboration and licensing agreement to develop and commercialize Antithrombin III (ATIII) as a treatment for sepsis. Grifols will supply ATIII and invest up to $25 million in clinical development, while Endpoint Health will conduct a Phase II trial using its AIbased companion diagnostic and hold global commercial rights (exChina).
Such initiatives enhance innovation, reduce financial burden, and enable faster market access, creating a robust pathway for expanded antithrombin use in critical care.
Regional Insights
The North American market is expanding due to advanced healthcare infrastructure, increased adoption of recombinant biologics, and high awareness of coagulation disorders. Robust diagnostic capabilities, favorable reimbursement frameworks, and proactive screening for thrombophilia further support market penetration. Growth is also driven by ongoing clinical trials and innovation in anticoagulant therapies. The presence of major biopharmaceutical players and collaborations with research institutions enhances the development pipeline. Increased surgical procedures, especially organ transplants and cardiovascular surgeries, are boosting the clinical demand for antithrombin supplementation across hospitals and specialty clinics.
U.s. Antithrombin Market Trends
- The U.S. market is driven by high thrombotic disease prevalence and advanced healthcare infrastructure. The FDA-approved recombinant antithrombin, ATryn by rEVO Biologics, is widely used in hereditary antithrombin deficiency cases. Increased surgical procedures and a strong focus on rare disease treatments also contribute to market growth. Moreover, significant R&D investments and government incentives support continuous innovation in biologic therapies targeting coagulation disorders.
- Canada’s antithrombin market is expanding due to improved diagnostic screening and public awareness about thrombosis-related risks. The Canadian Blood Services facilitates the distribution of plasma-derived antithrombin, especially in critical care and surgical settings. Additionally, national health programs support the early detection of hereditary deficiencies. Ongoing collaboration between hospitals and biotech firms further enhances treatment access, with emphasis on cost-effective biologics in managing congenital and acquired antithrombin deficiencies.
Asia-Pacific: Significantly Growing Region
The Asia Pacific market is rapidly evolving due to rising awareness of thrombosis-related conditions and expanding diagnostic infrastructure. Growing healthcare investments, coupled with increasing affordability of biologics, support broader market accessibility. Demand is being fueled by rising surgical volumes and greater use of anticoagulants in intensive care and transplant settings. Government initiatives promoting rare disease management and improved pharmacovigilance are also contributing factors. The increasing presence of biopharma companies in local markets is fostering technology transfer, aiding in the production and distribution of cost-effective recombinant antithrombin products.
- China’s antithrombin market is witnessing growth due to increasing awareness of thrombotic disorders and rising surgical volumes. The government's focus on rare disease diagnosis has improved accessibility to treatments like recombinant antithrombin. For example, regulatory reforms under China’s National Rare Disease Registry System are accelerating approvals. Additionally, domestic biopharma players like WuXi Biologics are entering the space, boosting local manufacturing and R&D.
- India’s market for antithrombin is expanding gradually, driven by growing cases of deep vein thrombosis and increased organ transplant procedures. Government programs like Ayushman Bharat have improved access to critical care, indirectly supporting anticoagulant use. For instance, tertiary hospitals such as AIIMS are adopting recombinant antithrombin for surgery-related coagulopathy. However, affordability remains a challenge, prompting interest in cost-effective biosimilars from Indian firms like Biocon and Intas.
Europe: Substantial Potential for Growth
Europe’s market is witnessing steady growth owing to stringent regulatory standards, strong emphasis on patient safety, and wide adoption of recombinant antithrombin therapies. The region benefits from structured healthcare systems, early disease screening protocols, and active surveillance of thrombotic disorders. High prevalence of rare bleeding disorders and a growing geriatric population enhance therapeutic demand. Research initiatives funded by the public and private sectors are accelerating innovation. Hospitals and specialized clinics increasingly prefer antithrombin in perioperative settings, especially in cardiovascular and orthopedic surgeries, reinforcing its importance in clinical anticoagulation protocols.
- Germany’s antithrombin industry benefits from a well-established healthcare system and strong pharmaceutical R&D. The country’s focus on rare disease management supports the use of antithrombin in hereditary deficiency cases. For instance, clinics affiliated with Charité – Universitätsmedizin Berlin are actively involved in clinical trials using recombinant antithrombin. Additionally, Germany’s emphasis on advanced surgical procedures drives demand for antithrombin during transplant and cardiac surgeries.
- The UK antithrombin market is driven by NHS initiatives to improve the diagnosis and treatment of thrombotic conditions. Growing awareness around hereditary thrombophilia has led to increased screening and therapeutic use of antithrombin. For example, the Royal Free London NHS Trust has adopted recombinant antithrombin in critical care settings. The UK’s support for orphan drugs and robust clinical research ecosystem also accelerates antithrombin adoption and innovation.
Market Segmentation
Source Insights
The recombinant goat milk segment is gaining prominence due to its innovative use in producing recombinant human antithrombin. This biotechnological approach involves genetically modified goats that secrete this molecule in their milk, enabling scalable and cost-effective production. Recombinant sources eliminate the risk of blood-borne pathogen transmission and offer improved consistency and purity compared to plasma-derived products. Companies like rEVO Biologics have commercialized such products, enhancing treatment accessibility for hereditary antithrombin deficiency and improving patient safety profiles globally.
Dosage Form Insights
The lyophilized powder segment is growing due to its enhanced stability and longer shelf life. This freeze-dried formulation of antithrombin allows for convenient storage and transportation, especially in regions with limited cold chain infrastructure. It is widely used in clinical settings where reconstitution before administration is feasible, such as in surgical procedures or intensive care. The segment’s reliability and ease of distribution make it a preferred choice among healthcare providers handling critical thrombosis-related interventions.
Application Insights
The therapeutics segment holds a significant share of the market, driven by its extensive use in treating hereditary antithrombin deficiency and preventing thromboembolic events. This molecule is also utilized during high-risk surgeries, such as cardiac and organ transplants, where anticoagulation is essential. Its application in managing sepsis-associated coagulopathy is also under investigation. As awareness increases and clinical guidelines evolve, the therapeutic use of antithrombin continues to expand, reinforcing its role as a critical biological agent in modern medicine.
End-User Insights
Hospitals represent the largest end-user segment in the market due to their central role in administering antithrombin for acute care settings. The product is frequently used in surgical theaters, intensive care units, and during high-risk deliveries. Hospitals are well-equipped with the infrastructure needed to handle both recombinant and plasma-derived forms of antithrombin. Additionally, increased hospital admissions related to cardiovascular disorders and thrombotic complications contribute to the rising demand, reinforcing the importance of this segment in overall market growth.
Company Market Share
Companies in the market for antithrombin are focusing on expanding their market share through strategic initiatives such as increasing investment in R&D, developing recombinant antithrombin therapies, and enhancing production capabilities. They are also pursuing regulatory approvals in emerging markets, forming partnerships with hospitals and research institutions, and expanding their presence in high-growth regions. Additionally, many are emphasizing awareness campaigns and early diagnostics to drive demand and boost long-term adoption of antithrombin treatments.
Grifols S.A.: Grifols S.A., headquartered in Barcelona, Spain, is a global healthcare company specializing in plasma-derived therapies. A key player in the antithrombin market, Grifols develops and manufactures biologic medicines to treat rare and chronic conditions, including antithrombin deficiency. With a strong portfolio of plasma-derived products, the company emphasizes innovation, research, and global plasma collection infrastructure. Grifols’ focus on quality and safety standards has positioned it as a trusted provider of antithrombin therapies across hospitals, surgical units, and critical care settings.
- In July 2025, Grifols announced a €160 million investment to build a new plasma fractionation plant in Lliçà de Vall (Barcelona), doubling its European processing capacity. Scheduled to be operational by 2030, the 80,000 m² facility will bolster supply of antithrombin and other plasma-derived therapies, create over 400 jobs, and integrate renewable energy systems.
List of Key and Emerging Players in Antithrombin Market
- Grifols S.A.
- CSL Behring
- Kedrion Biopharma
- Shire (Takeda Pharmaceutical Company Limited)
- Octapharma AG
- LEO Pharma A/S
to learn more about this report Download Market Share
Recent Developments
- June 2025- Sysmex received FDA 510(k) clearance for its CN‑6000 automated coagulation analyzer, which integrates an Innovance Antithrombin assay. This innovation supports high-throughput clinical lab monitoring of antithrombin levels, essential for safe management of patients on antithrombin-targeting therapies like Qfitlia, enhancing accuracy and workflow efficiency in modern coagulation diagnostics.
- March 2025- The FDA approved Qfitlia (fitusiran) for prophylaxis in hemophilia A and B (with or without inhibitors). This first-in-class antithrombin-lowering siRNA therapy offers approximately 90% bleeding reduction with subcutaneous dosing every two months. Co-developed by Alnylam and Sanofi, its U.S. launch marks a pivotal shift in hemophilia treatment.
- March 2025- Siemens Healthineers' Innovance Antithrombin assay received FDA clearance as the first companion diagnostic for Qfitlia. This diagnostic enables precise monitoring of antithrombin levels in patients with hemophilia, ensuring safe and effective dosing. The approval supports personalized treatment and marks a significant advancement in the antithrombin therapeutic ecosystem.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 422.32 Million |
| Market Size in 2025 | USD 443.23 Million |
| Market Size in 2033 | USD 652.35 Million |
| CAGR | 4.95% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Source, By Dosage Form, By Application, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Antithrombin Market Segments
By Source
- Human
- Recombinant Goat Milk
By Dosage Form
- Lyophilized Powder
- Liquid Form
By Application
- Therapeutics
- Research
- Diagnostics
- Others
By End User
- Hospitals
- Specialty Clinics
- Academic & Research Institutes
- Diagnostic Laboratories
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































