Artificial Lung Market Size, Share & Trends Analysis Report By Product Type (Extracorporeal Membrane Oxygenation (ECMO) Systems, Paracorporeal Artificial Lungs, Intracorporeal Artificial Lungs, Membrane Oxygenators, Portable Artificial Lungs), By Application (Bridge-to-Transplantation, Bridge-to-Recovery, ECMO-assisted Ventilation, Pulmonary Rehabilitation, Long-term Support for Chronic Lung Diseases), By Distribution Channel (Hospital-based Procurement, Direct Tenders and Contracts, Group Purchasing Organizations (GPOs), Online Procurement Platforms, Third-party Distributors), By End-User (Tertiary Care Hospitals, Transplant Centers, Cardiac and Pulmonary ICUs, Research Institutions and Clinical Trial Sites, Specialty Respiratory Clinics) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Artificial Lung Market Overview
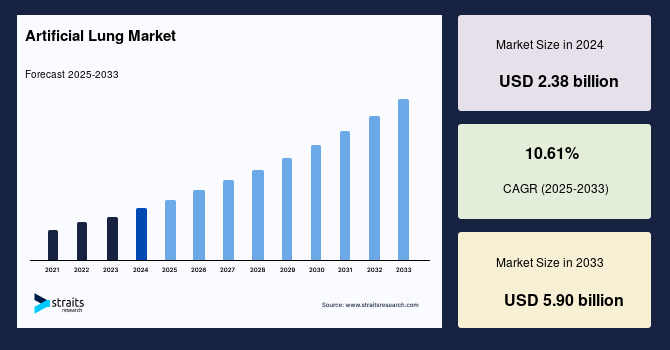
The global artificial lung market size was valued at USD 2.38 billion in 2024 and is projected to grow from USD 2.63 billion in 2025 to reach USD 5.90 billion by 2033, growing at a CAGR of 10.61% during the forecast period (2025–2033). The growth of the market is driven by the rising incidence of chronic respiratory diseases, increasing demand for advanced respiratory support systems, ongoing advancements in extracorporeal life support technologies, and revolutionised critical respiratory care delivery.
Key Market Indicators
- North America dominated the artificial lung industry and accounted for a 43% share in 2024.
- Based on product type, the ECMO systems segment dominated the market in 2024 due to their effectiveness in treating severe respiratory or cardiopulmonary failure. Their modular design and ability to provide sustained support when conventional ventilation fails drive widespread adoption in hospitals.
- Based on application, the bridge-to-transplantation segment is witnessing steady growth, driven by these systems providing vital support for patients awaiting donor lung availability.
- Based on distribution channel, the hospital-based procurement segment dominated the global market in 2024, owing to specialized infrastructure needs and demand for reliable performance and support.
Market Size & Forecast
- 2024 Market Size: USD 2.38 billion
- 2033 Projected Market Size: USD 5.90 billion
- CAGR (2025–2033): 10.61%
- Largest market in 2024: North America
- Fastest-growing region: Asia Pacific
Artificial lungs are crucial for treating conditions like ARDS and COPD and supporting lung transplant patients. Widely used in ICUs, surgical centres, and transplant programs, their demand is rising due to increasing healthcare spending, awareness of organ support technologies, and broader use of ECMO in critical care, especially post-COVID-19. Technological innovations in membrane oxygenators, AI-based monitoring, and biocompatible materials are enhancing safety and efficiency, while research into bioartificial lungs and regenerative scaffolds is expanding future possibilities.
Latest Market Trend
Portability and Defence-Driven Innovation
The artificial lung market is transforming strategically as medtech companies respond to growing demand for portable, autonomous, and AI-integrated respiratory systems. This shift reshapes clinical expectations and reflects broader interest from defence sectors and critical care innovators seeking resilient, field-deployable organ support technologies.
- For instance, in December 2024, Johns Hopkins Medicine secured over $18 million in DARPA funding to lead the development of a next-generation portable ECMO-based artificial lung system designed for use in military trauma settings. In collaboration with Carnegie Mellon University and Vanderbilt University, the project aims to deliver a battery-powered, closed-loop device capable of functioning independently from ICU infrastructure.
As healthcare and defence sectors converge around shared goals of resilience, portability, and autonomy, artificial lung manufacturers who embrace this direction are well-positioned for long-term growth.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 2.38 billion |
| Estimated 2025 Value | USD 2.63 billion |
| Projected 2033 Value | USD 5.90 billion |
| CAGR (2025-2033) | 10.61% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Getinge AB, Xenios AG (Fresenius Medical Care), Medtronic plc, Abbott Laboratories, Abiomed (Johnson and Johnson MedTech) |
to learn more about this report Download Free Sample Report
Artificial Lung Market Driver
Portable ECMO Systems Revolutionise Critical Respiratory Care Delivery
A major driver of the artificial lung market is the increasing adoption of compact, transport-ready extracorporeal membrane oxygenation (ECMO) systems, enabling advanced respiratory support beyond traditional ICU settings. As healthcare systems face rising demand for flexible, mobile life-support technologies, innovation in portable artificial lung devices is reshaping critical care and emergency response.
- For instance, in December 2024, Yale New Haven Health became the first health system in the United States to deploy Medtronic’s FDA-approved VitalFlow ECMO System for inter-hospital patient transport. The system, designed for longer-term respiratory and circulatory support, was successfully used during a transfer from Greenwich Hospital to Yale New Haven Hospital.
As hospitals, trauma centers, and transplant networks increasingly seek scalable, transportable solutions to support patients with acute respiratory distress or chronic pulmonary failure, the demand for next-generation artificial lung systems is accelerating.
Market Restraint
High Costs and Complexity Hinder Widespread Clinical Adoption
The high cost and technical complexity of extracorporeal membrane oxygenation (ECMO) and other artificial lung systems restrain market growth. Their deployment requires specialised infrastructure, continuous monitoring, and trained personnel, factors that limit adoption across lower-resource hospitals and rural healthcare settings. Artificial lungs demand intensive staffing, including perfusionists and critical care teams, and involve substantial maintenance and consumables costs.
Additionally, procurement budgets, especially in public hospitals and developing markets, often cannot accommodate these capital and operational expenses, reducing accessibility for broader patient populations. Until cost-effective, user-friendly, and low-maintenance artificial lung solutions are widely commercialised, market growth may remain concentrated within high-end tertiary care and academic institutions, limiting scale in mainstream respiratory care delivery.
Market Opportunity
Academic Innovation Propels the Development of Portable, Automated ECMO Systems
A compelling opportunity in the artificial lung sector emerges from university-led engineering projects focused on miniaturisation and automation, spotlighting new pathways for patient-friendly, mobile respiratory support solutions.
- In May 2025, a team of biomedical engineering students at Vanderbilt University, working in collaboration with Vanderbilt University Medical Center, unveiled a senior-design prototype for an automated portable ECMO device. This system integrates an oxygen concentrator, capnography-based CO₂ sensing, and servo-motor–driven controls to dynamically adjust gas exchange, reducing reliance on clinician intervention and enabling enhanced mobility and speed during critical situations.
With increasing clinical validation and potential pathways for commercialization, such academic innovations offer a scalable opportunity: artificial lung developers can collaborate with universities and startups to accelerate next-gen portable respiratory technologies.
Regional Insights
North America dominated the artificial lung industry in 2024, accounting for a 43% market share. The region leads the artificial lung market, supported by its advanced healthcare infrastructure and strong integration of life-saving technologies into intensive care units. It demonstrates high clinical adoption of artificial lungs, particularly in managing severe respiratory failure and cardiopulmonary conditions. Healthcare facilities consistently prioritise innovation, reliability, and regulatory compliance, fostering the early use of extracorporeal and implantable artificial lung systems. In addition, hospitals and research institutions in the region maintain established protocols for patient monitoring, surgical procedures, and post-implant care, enabling smoother integration of artificial lung devices.
- The U.S remains the largest market for artificial lungs, driven by its advanced critical care ecosystem and robust investment in life-saving medical technologies. Major hospitals and trauma centres routinely deploy ECMO systems and implantable lung support devices in adult and pediatric ICUs. Regulatory support from agencies like the FDA accelerates market access for innovative artificial lung solutions through breakthrough designations and fast-track approvals. Academic medical centers are conducting leading-edge clinical trials to test portable and long-term artificial lung technologies in patients awaiting lung transplants.
- Canada's artificial lung industry is gaining momentum, supported by rising demand in tertiary hospitals and organ transplant centers. The country's universal healthcare system prioritises access to advanced cardiopulmonary support, gradually integrating ECMO and artificial lung devices into standard intensive care protocols. Hospitals across provinces are upgrading their cardiothoracic equipment with more compact and biocompatible lung support systems to improve survival in severe ARDS and cardiac failure cases. Government-funded research initiatives also explore artificial lung solutions for mobile critical care units.
Asia-Pacific Market Trends
Asia-Pacific is emerging as a high-potential region for the artificial lung industry, primarily driven by growing investments in healthcare modernisation and critical care capabilities. Medical institutions across the region are increasingly exploring artificial lung technologies to address complex respiratory conditions and bridge gaps in organ availability. The adoption of extracorporeal and portable systems is gaining momentum as healthcare providers prioritise versatile solutions that support emergency care and long-term respiratory support. Local medical device manufacturing and clinical research advancements also contribute to increased accessibility and cost-efficiency.
- China is witnessing rapid growth in the market for artificial lung due to expanding ICU capacity and aggressive healthcare reforms. Top-tier hospitals are adopting ECMO technologies and beginning pilot use of compact artificial lung systems in both adult and neonatal critical care settings. The rise in chronic respiratory illnesses has prompted national health authorities to prioritise advanced respiratory care in regional and provincial hospitals. Local medical device manufacturers are partnering with global firms to co-develop artificial lung systems that comply with Chinese and international standards. China’s ageing population and rising air pollution levels contribute to a growing patient base for lung support therapies.
- India's market for artificial lung is evolving steadily, driven by improvements in emergency medicine infrastructure and a rising number of critical care centers. While still in early adoption stages, major hospitals and private medical institutes are beginning to deploy ECMO support in severe ARDS and cardiac arrest cases. A growing awareness of transplant alternatives has sparked interest in temporary artificial lung support, especially in high-volume cardiothoracic departments. Government initiatives to modernise ICU facilities are indirectly accelerating demand for life-support innovations. With increasing interest from international NGOs, artificial lung use in humanitarian disaster response is emerging as a potential application segment.
Europe Market Trends
Europe is witnessing steady growth in the artificial lung market due to its widespread adoption of advanced medical technologies and emphasis on quality patient care. Regionally, Healthcare systems increasingly incorporate artificial lung support as standard treatment strategies in critical care units. Hospitals and clinics maintain high operational standards, driving the demand for reliable, biocompatible artificial lung devices that deliver consistent performance. Growing emphasis on safety, patient outcomes, and post-operative support encourages healthcare providers to integrate such technologies into temporary and long-term respiratory management strategies.
- Germany is a frontrunner in artificial lung innovation, thanks to its highly structured healthcare system and strong emphasis on clinical precision. The country's stringent medical device regulations ensure high-quality, biocompatible systems with advanced oxygenation capabilities. German manufacturers are developing next-generation artificial lungs with optimised gas exchange and reduced hemolysis, tailored for both short- and long-term applications. Moreover, the country’s expanding geriatric population has prompted greater investment in critical care and post-operative respiratory support, further driving market growth.
- The UK artificial lung industry is expanding, supported by ongoing healthcare reforms and renewed focus on intensive care excellence. Hospitals incorporate portable and modular artificial lung systems into their ECMO services, particularly in urban trauma and transplant centers. National funding bodies are investing in research projects that explore hybrid devices combining respiratory and cardiac support functions. Additionally, clinical training programs across NHS facilities are upskilling healthcare professionals in advanced extracorporeal therapies.
Product Type Insights
Extracorporeal Membrane Oxygenation (ECMO) Systems dominate the market due to their proven efficacy in supporting critically ill patients with severe respiratory or cardiopulmonary failure. ECMO systems function by externally oxygenating blood and removing carbon dioxide, temporarily taking over the gas exchange function of the lungs. Their modular design, comprising pumps, oxygenators, and control consoles, offers adaptability across different clinical needs, from acute respiratory distress syndrome (ARDS) to post-cardiotomy recovery. Hospitals value ECMO for its ability to provide sustained support over days or weeks, particularly when conventional ventilation fails.
Application Insights
Bridge-to-transplantation remains the most dominant application, as these systems provide vital support for patients awaiting donor lung availability. Their use significantly enhances survival rates and quality of life during the critical pre-transplant phase. Hospitals prioritise devices with long-duration support capability, low thrombogenic potential, and easy integration with extracorporeal life support (ECLS) protocols. Due to organ shortages and lengthy waiting times, artificial lungs for bridge-to-transplantation have become an integral part of transplant centre workflows, with dedicated protocols and specialised staff managing patients on long-term mechanical pulmonary support.
Distribution Channel Insights
Hospital-based procurement channels dominate the market, primarily due to the highly specialised nature of these devices and the procedural infrastructure they require. These transactions typically occur through direct supply agreements, negotiated contracts, or tenders coordinated by biomedical engineering and procurement departments within tertiary care hospitals and transplant centers. Given artificial lungs' critical role in life-threatening cases, hospitals demand consistent service reliability, performance validation, and quick-response maintenance capabilities that general retail or third-party distributors cannot adequately offer.
End-User Insights
Tertiary care hospitals and transplant centers are the primary end users of artificial lungs, given their advanced infrastructure and multidisciplinary teams required for managing patients on prolonged extracorporeal support. These institutions house specialised cardiac and pulmonary intensive care units capable of performing high-risk procedures such as ECMO-assisted ventilation and pre-transplant stabilisation. Artificial lungs in these settings are utilised under tightly controlled protocols involving cardiothoracic surgeons, pulmonologists, perfusionists, and ICU nurses trained in mechanical respiratory systems. 24/7 emergency response units and on-site diagnostics further enable safe, continuous monitoring and adjustment of lung support systems.
Company Market Share
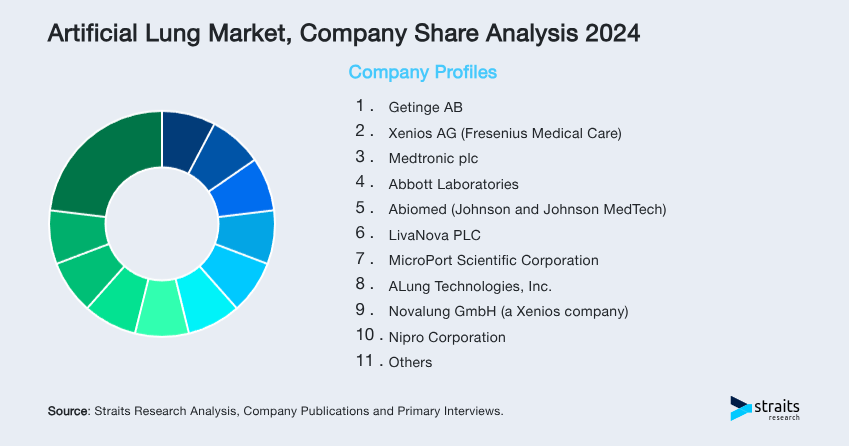
The artificial lung market is moderately consolidated, with a small group of medtech leaders driving innovation, manufacturing, and global distribution. As of mid-2025, the top five players, Getinge AB, Xenios AG (Fresenius Medical Care), Medtronic plc, Abbott Laboratories, and Abiomed (Johnson and Johnson MedTech), collectively account for nearly 42% of global revenue share. These firms dominate due to advanced ECMO platforms, regulatory approvals, long-standing clinical partnerships, and integrated post-operative support solutions.
Getinge AB is a Sweden-based global medical technology company founded in 1904, specializing in advanced healthcare solutions for intensive care, cardiovascular procedures, surgical workflows, and life sciences. With operations in over 135 countries, it is best known for its Cardiohelp ECMO system and other life-support technologies used in hospitals and transport settings.
- As of July 2025, Getinge AB remains a global leader in extracorporeal life support systems, most notably with its Cardiohelp ECMO platform. The Cardiohelp system is widely deployed in tertiary care centres and academic hospitals for venovenous (VV) and venovenous (VV) ECMO support. Its compact design, portability, and integrated monitoring capabilities make it suitable for ICUs, operating rooms, and inter-hospital transport.
List of Key and Emerging Players in Artificial Lung Market
- Getinge AB
- Xenios AG (Fresenius Medical Care)
- Medtronic plc
- Abbott Laboratories
- Abiomed (Johnson and Johnson MedTech)
- LivaNova PLC
- MicroPort Scientific Corporation
- ALung Technologies, Inc.
- Novalung GmbH (a Xenios company)
- Nipro Corporation
- Hemovent GmbH
- Braile Biomédica
- MC3 Cardiopulmonary
to learn more about this report Download Market Share
Recent Developments
- September 2025: ALung Technologies began commercial development of its next-generation Hemolung Respiratory Assist System (RAS), designed for portable and integrated artificial lung support, while Michigan Instruments launched advanced artificial lung simulators for training, testing, and calibration, reflecting major advancements in the artificial lung market.
- September 2025: Michigan Instruments launched advanced artificial lung simulators for adult and infant models, designed to train healthcare professionals, test and calibrate ventilators, and simulate patient conditions, enhancing respiratory care and device development.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 2.38 billion |
| Market Size in 2025 | USD 2.63 billion |
| Market Size in 2033 | USD 5.90 billion |
| CAGR | 10.61% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product Type, By Application, By Distribution Channel, By End-User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Artificial Lung Market Segments
By Product Type
- Extracorporeal Membrane Oxygenation (ECMO) Systems
- Paracorporeal Artificial Lungs
- Intracorporeal Artificial Lungs
- Membrane Oxygenators
- Portable Artificial Lungs
By Application
- Bridge-to-Transplantation
- Bridge-to-Recovery
- ECMO-assisted Ventilation
- Pulmonary Rehabilitation
- Long-term Support for Chronic Lung Diseases
By Distribution Channel
- Hospital-based Procurement
- Direct Tenders and Contracts
- Group Purchasing Organizations (GPOs)
- Online Procurement Platforms (limited role)
- Third-party Distributors (emerging in select regions)
By End-User
- Tertiary Care Hospitals
- Transplant Centers
- Cardiac and Pulmonary ICUs
- Research Institutions and Clinical Trial Sites
- Specialty Respiratory Clinics
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































