Autologous Cell Therapy Market Size, Share & Trends Analysis Report By Cell Type (Hematopoietic Stem Cells (HSCs), Mesenchymal Stem Cells (MSCs), Chondrocytes, Fibroblasts, Keratinocytes, Other Autologous Cells), By Source (Epidermis, Bone Marrow, Mesenchymal Stem Cells, Haematopoietic Stem Cells, Chondrocytes, Others), By Application (Cancer, Cardiovascular Disorders, Neurodegenerative Disorders, Autoimmune Disorders, Orthopedics, Wound Healing, Others), By End-User (Hospitals & Clinics, Ambulatory Centers, Academics & Research, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Autologous Cell Therapy Market Size
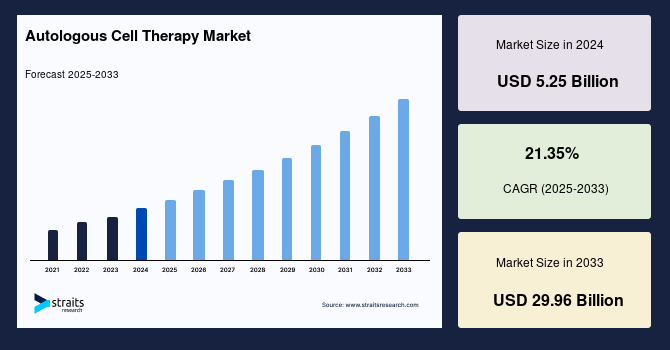
The global autologous cell therapy market size was valued at USD 5.25 billion in 2024 and is projected to grow from USD 6.37 billion in 2025 to USD 29.96 billion by 2033, growing at a CAGR of 21.35% during the forecast period (2025–2033).
One of the key drivers of the global autologous cell therapy market is the growing demand for personalized and precision medicine. As healthcare systems shift toward individualized treatment strategies, autologous cell therapy aligns perfectly with this paradigm by utilizing a patient's own cells to develop targeted therapies. This significantly enhances treatment efficacy and reduces the risk of adverse immune responses.
Another critical aspect propelling market growth is the rising incidence of genetic disorders and autoimmune diseases. Conditions such as multiple sclerosis and certain inherited anemias are increasingly being explored for treatment through autologous stem cell interventions, as these therapies offer potential long-term remission or disease-modifying effects. Furthermore, increasing awareness and patient preference for minimally invasive procedures often associated with autologous treatments are pushing healthcare providers and institutions to adopt these advanced techniques, thereby contributing to market expansion.
Autologous Cell Therapy Market Trends
Advancements in Cell Processing Technologies
A significant trend shaping the global market is the advancement in cell processing technologies, which are improving the efficiency, safety, and scalability of personalized treatments. Automated bioreactors, closed-system processing, and AI-integrated monitoring are helping reduce contamination risks and ensure consistent product quality. These innovations are particularly important for autologous therapies, which require precise handling of patient-derived cells.
- Toward the middle of this transformation, a notable development came from Kyoto University's CiRA Foundation, which began the automated production of autologous induced pluripotent stem cells (iPSCs) in April 2025. Using a German-built immune-cell processing system, they can now convert a patient's blood into iPSCs within a month, cutting both time and cost. These cells are then differentiated into heart or nerve cells for study and therapy.
Such advancements are accelerating clinical translation and improving access to autologous treatments.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 5.25 Billion |
| Estimated 2025 Value | USD 6.37 Billion |
| Projected 2033 Value | USD 29.96 Billion |
| CAGR (2025-2033) | 21.35% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
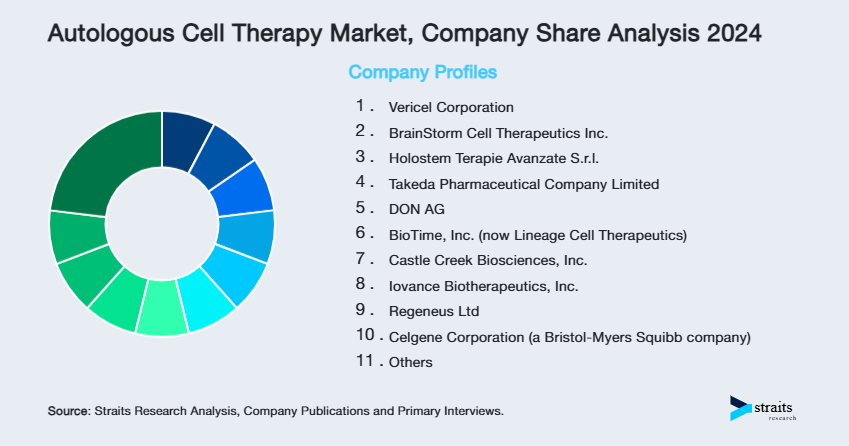
| Key Market Players | Vericel Corporation, BrainStorm Cell Therapeutics Inc., Holostem Terapie Avanzate S.r.l., Takeda Pharmaceutical Company Limited, DON AG |
to learn more about this report Download Free Sample Report
Autologous Cell Therapy Market Drivers
Increasing Prevalence of Chronic and Degenerative Diseases
The rising burden of chronic and degenerative diseases is a key driver fostering the growth of the global autologous cell therapy industry. These conditions often require long-term treatment and regenerative approaches, making autologous therapies a suitable solution.
- According to recent data, neurodegenerative diseases like Parkinson's are escalating, with cases growing from 450,000 in 1992 to 1.34 million in 2021 and expected to reach 25.2 million globally by 2050 a 112% increase. Similarly, the number of people living with diabetes has risen sharply from 200 million in 1990 to 830 million by 2022, affecting nearly 1 in 7 adults globally.
These growing statistics underscore the urgent need for personalized and regenerative treatments. Autologous therapies, by utilizing a patient's own cells, offer safer and more effective alternatives for managing such complex conditions.
Market Restraining Factors
High Cost and Complex Manufacturing
One of the primary restraints in the global autologous cell therapy market is the high cost and complex manufacturing process. Each therapy must be customized for individual patients, requiring advanced infrastructure, skilled personnel, and time-consuming procedures. This personalization limits scalability and significantly increases production costs compared to allogeneic or conventional treatments.
Moreover, maintaining strict quality control and compliance with regulatory standards adds further financial and logistical burdens. These factors make the therapies less accessible, especially in low- and middle-income regions, hindering broader market adoption. Addressing these manufacturing challenges remains crucial for improving affordability and expanding the market reach of autologous cell therapies.
Market Opportunities
Strategic Collaborations and R&d Funding
Strategic collaborations and increased R&D funding offer immense growth potential for the global market for autologous cell therapy. These alliances foster innovation, accelerate clinical translation, and strengthen commercialization pathways. By leveraging shared expertise and infrastructure, companies can navigate regulatory challenges and scale complex manufacturing processes.
- For instance, in March 2025, AstraZeneca announced the acquisition of biotech firm EsoBiotec for up to $1 billion, including an initial $425M and $575M in milestone payments. The deal aims to scale autologous cell therapy development particularly in oncology by combining EsoBiotec's cell-engineering expertise with AstraZeneca's global development and manufacturing infrastructure.
Such high-value collaborations highlight the growing investor confidence in autologous therapies. Additionally, support from government-backed R&D programs and venture capital funding further fuels progress. These combined efforts are paving the way for broader therapeutic applications and faster patient access to personalized, cell-based treatments.
Regional Insights
North America is witnessing robust growth in the autologous cell therapy market due to high healthcare expenditure, a well-established regulatory framework, and increasing clinical trials in regenerative medicine. The region benefits from a robust presence of research institutions and advanced cell therapy manufacturing infrastructure. Additionally, favorable reimbursement policies and growing awareness among clinicians and patients are accelerating adoption. The integration of cutting-edge technologies in cell processing and cryopreservation further supports the regional expansion of autologous therapies, particularly in treating orthopedic, cardiovascular, and neurological disorders.
U.s. Autologous Cell Therapy Market Trends
- The United States market is advancing rapidly due to high R&D investments and robust clinical infrastructure. FDA-approved therapies like Yescarta and Abecma exemplify growing acceptance. Leading institutions like MD Anderson and the Mayo Clinic are spearheading clinical trials. Strong support from organizations like the NIH and favorable reimbursement policies further bolster market expansion across oncology and regenerative medicine applications.
- The Canadian autologous cell therapy market is gaining traction with increased government funding and collaborations. Projects like CellCAN, supported by CIHR and Genome Canada, aim to standardize cell therapy manufacturing. Institutions like the Ottawa Hospital Research Institute are actively involved in clinical research. The country's streamlined regulatory framework via Health Canada facilitates the rapid development of patient-specific therapies, especially for orthopedic and autoimmune conditions.
Asia-Pacific Autologous Cell Therapy Market Trends
Asia Pacific is emerging as a promising market for autologous cell therapy, supported by expanding healthcare infrastructure, surging medical tourism, and increasing government initiatives in regenerative medicine. A surge in chronic diseases and a growing focus on stem cell-based research are enhancing the region's market potential. Local biotech firms are actively investing in developing cost-effective autologous therapies tailored to regional healthcare needs. Furthermore, improvements in clinical trial regulations and investments in specialized cell therapy facilities are fostering faster product development and market penetration across therapeutic areas.
- China's autologous cell therapy market is witnessing rapid growth driven by strong government support and increasing investments in regenerative medicine. For example, the 14th Five-Year Plan emphasizes biotech innovation, benefiting cell therapy development. Companies like Fosun Pharma and Immunochina are actively engaged in clinical trials of autologous CAR-T and stem cell therapies, positioning China as a competitive hub for personalized cell-based treatments in oncology and orthopedics.
- India's autologous cell therapy industry is expanding due to rising medical tourism, growing chronic disease burden, and evolving regulatory clarity. Institutions like AIIMS and Manipal Hospitals are conducting stem cell-based autologous treatments for orthopedic and neurological conditions. Moreover, government initiatives such as the National Guidelines for Stem Cell Research promote safe therapy development. India's cost-effective healthcare system makes it an attractive destination for autologous cell therapy innovations and services.
Europe Autologous Cell Therapy Market Trends
Europe's market for autologous cell therapy market is driven by the region's emphasis on innovative healthcare solutions and strong government support for advanced therapy medicinal products (ATMPs). Increased funding for biomedical research and streamlined regulatory pathways have facilitated clinical adoption. The surging geriatric population and rising incidence of chronic diseases are increasing the demand for regenerative treatments. Collaborations between academia and biopharma companies are fueling clinical research and therapy development. Moreover, expanding biomanufacturing capabilities and increased public awareness about personalized medicine are propelling the regional growth of autologous cell-based interventions.
- Germany's market for autologous cell therapy is expanding due to its advanced biotechnology infrastructure and supportive reimbursement policies. The country's focus on regenerative medicine is evident in projects like the Fraunhofer Institute's stem cell therapy research. Moreover, clinical trials in orthopedic and cardiac applications are increasing. Government and EU funding further accelerates innovation, positioning Germany as a leading hub for autologous regenerative therapies in Europe.
- UK's autologous cell therapy industry is driven by strong academic research, NHS integration, and government support through agencies like Innovate UK. Institutions such as the Cell and Gene Therapy Catapult foster clinical adoption and commercialization. Notably, King's College London is leading trials on autologous stem cells for spinal cord injury. The UK's regulatory clarity and focus on personalized medicine strengthen its role in advancing autologous cell therapy solutions.
Cell Type Insights
The hematopoietic stem cells (HSCs) segment holds a significant share in the autologous cell therapy market due to their well-established use in treating blood-related disorders and cancers, especially leukemia and lymphoma. HSCs possess the unique ability to self-renew and differentiate into all blood cell types, making them crucial for bone marrow transplants. Their clinical efficacy, particularly in autologous transplantation, minimizes the risk of graft-versus-host disease. Additionally, ongoing advancements in harvesting and cryopreservation techniques, along with strong clinical research support, are further strengthening the adoption of HSC-based therapies in both oncology and regenerative medicine.
Source Insights
Bone marrow is the dominant source segment in the market owing to its rich reservoir of stem cells, particularly hematopoietic and mesenchymal stem cells. It remains a preferred source for autologous transplantation in treating hematological malignancies, autoimmune diseases, and orthopedic conditions. The ease of access and high concentration of viable cells make bone marrow a reliable and effective source. Furthermore, standardized protocols and improved extraction technologies have enhanced procedural safety and efficiency. Its long-standing clinical acceptance and successful therapeutic outcomes continue to drive its dominance in the autologous cell therapy landscape.
Application Insights
Cancer is the leading application segment in the autologous cell therapy market, primarily due to the high global burden of cancer and the increasing demand for personalized treatments. Autologous cell therapies, such as autologous hematopoietic stem cell transplantation (HSCT), are widely used in treating hematologic malignancies like lymphoma, leukemia, and multiple myeloma. These therapies improve survival rates while reducing immunological complications. Additionally, innovations in tumor-infiltrating lymphocytes (TILs) and dendritic cell-based therapies are expanding autologous approaches in solid tumors. The shift toward individualized cancer care and rising clinical success stories continues to propel growth in this segment.
End-User Insights
Hospitals and clinics dominate the end-user segment of the market due to their advanced infrastructure, skilled medical personnel, and access to cutting-edge therapeutic technologies. These settings facilitate complex cell extraction, processing, and re-infusion procedures under stringent regulatory and safety standards. Moreover, the rising number of specialized oncology and regenerative medicine centers within hospitals enhances patient access to autologous treatments. Clinical trials, post-treatment monitoring, and emergency care are also efficiently handled in these facilities. Their integral role in diagnosis, therapy administration, and follow-up care positions hospitals and clinics as the primary hubs for autologous cell therapy delivery.
Company Market Share
Companies in the autologous cell therapy market are focusing on expanding their clinical pipelines, investing in advanced manufacturing platforms, and forming strategic partnerships with research institutions. They are also working to streamline regulatory approvals and enhance scalability through automation and point-of-care solutions. These efforts aim to improve treatment accessibility, reduce costs, and accelerate commercialization, thereby strengthening their position and expanding their share in the growing market.
BrainStorm Cell Therapeutics Inc.: BrainStorm is a clinical-stage biotechnology company established in 2000 and based in New York. It is committed to advancing innovative treatments aimed at addressing rare diseases and unmet medical needs. The company is a specialized autologous cell therapy firm focusing on neurodegeneration, with NurOwn as its flagship program. Backed by growing biomarker evidence and regulatory endorsement for confirmatory trials, it is moving forward with robust manufacturing partnerships.
- In April 2025, BrainStorm Cell Therapeutics Inc. submitted an amendment to its Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) for NurOwn® the company's autologous mesenchymal stem cell therapy aimed at treating amyotrophic lateral sclerosis (ALS). This key development paves the way for the launch of BrainStorm's much-anticipated Phase 3b clinical trial, which was developed in collaboration with the FDA as part of a Special Protocol Assessment (SPA).
List of Key and Emerging Players in Autologous Cell Therapy Market
- Vericel Corporation
- BrainStorm Cell Therapeutics Inc.
- Holostem Terapie Avanzate S.r.l.
- Takeda Pharmaceutical Company Limited
- DON AG
- BioTime, Inc. (now Lineage Cell Therapeutics)
- Castle Creek Biosciences, Inc.
- Iovance Biotherapeutics, Inc.
- Regeneus Ltd
- Celgene Corporation (a Bristol-Myers Squibb company)
- Novartis AG
- Pharmicell Co., Ltd.
- Medipost Co., Ltd.
to learn more about this report Download Market Share
Recent Developments
- June 2025- MaxCyte, a prominent company specializing in cell engineering and offering innovative platform technologies that support the discovery, development, and commercialization of next-generation cell therapies, and Ori Biotech, a recognized leader in advanced manufacturing technologies for cell and gene therapies (CGT), announced a collaboration aimed at enhancing manufacturing efficiency and expanding usage of autologous cellular therapies. This partnership leverages combined expertise in scalable, closed-loop production.
- June 2025- Limula launched beta testing for its fully automated autologous cell-processing platform, designed to simplify cell culture, separation, and formulation in a single, compact system. The founders intend to make personalized therapies affordable and widely accessible.
- April 2025- The FDA approved Zevaskyn (pz‑cel) the first autologous, cell-based gene therapy for recessive dystrophic epidermolysis bullosa (RDEB), a rare degenerative skin disorder. This milestone underscores growing recognition of autologous therapies as viable solutions for severe chronic conditions.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 5.25 Billion |
| Market Size in 2025 | USD 6.37 Billion |
| Market Size in 2033 | USD 29.96 Billion |
| CAGR | 21.35% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Cell Type, By Source, By Application, By End-User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Autologous Cell Therapy Market Segments
By Cell Type
- Hematopoietic Stem Cells (HSCs)
- Mesenchymal Stem Cells (MSCs)
- Chondrocytes
- Fibroblasts
- Keratinocytes
- Other Autologous Cells
By Source
- Epidermis
- Bone Marrow
- Mesenchymal Stem Cells
- Haematopoietic Stem Cells
- Chondrocytes
- Others
By Application
- Cancer
- Cardiovascular Disorders
- Neurodegenerative Disorders
- Autoimmune Disorders
- Orthopedics
- Wound Healing
- Others
By End-User
- Hospitals & Clinics
- Ambulatory Centers
- Academics & Research
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Dhanashri Bhapakar
Senior Research Associate
Dhanashri Bhapakar is a Senior Research Associate with 3+ years of experience in the Biotechnology sector. She focuses on tracking innovation trends, R&D breakthroughs, and market opportunities within biopharmaceuticals and life sciences. Dhanashri’s deep industry knowledge enables her to provide precise, data-backed insights that help companies innovate and compete effectively in global biotech markets.










































