Bioconvergence Technology Market Size, Share & Trends Analysis Report By Convergence Technology (Bioelectronics, Synthetic Biology, Biophotonics, Nano-bio Interfaces, 3D Bioprinting & Tissue Engineering, Bio-AI Platforms, Others), By Application (Diagnostics & Imaging, Drug Discovery & Development, Precision & Personalized Medicine, Regenerative Medicine & Tissue Engineering, Healthcare Analytics & Decision Support, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Bioconvergence Technology Market Overview
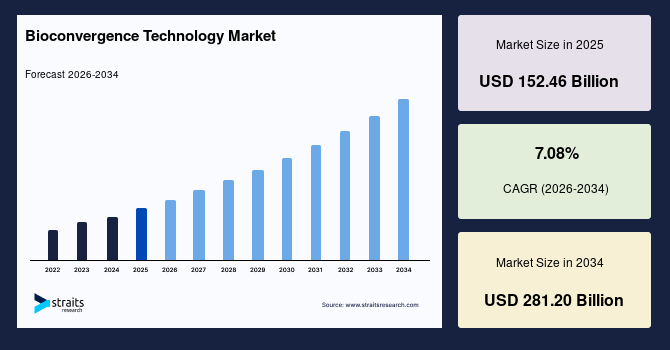
The global bioconvergence technology market size is valued at USD 152.46 billion in 2025 and is estimated to reach USD 281.20 billion by 2034, growing at a CAGR of 7.08% during the forecast period. The substantial market growth is attributed to the integration of nanotechnology and advanced computing to develop novel healthcare solutions.
Key Market Trends & Insights
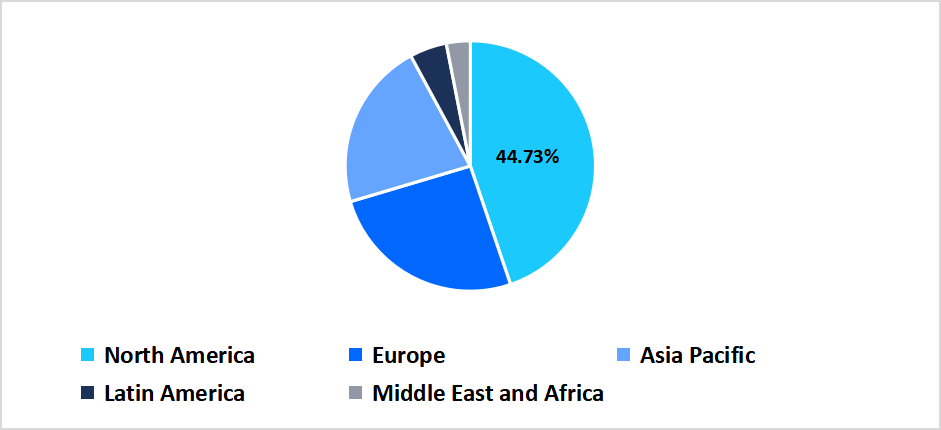
- North America held a dominant share of the global market, accounting for 44.73% in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 38%.
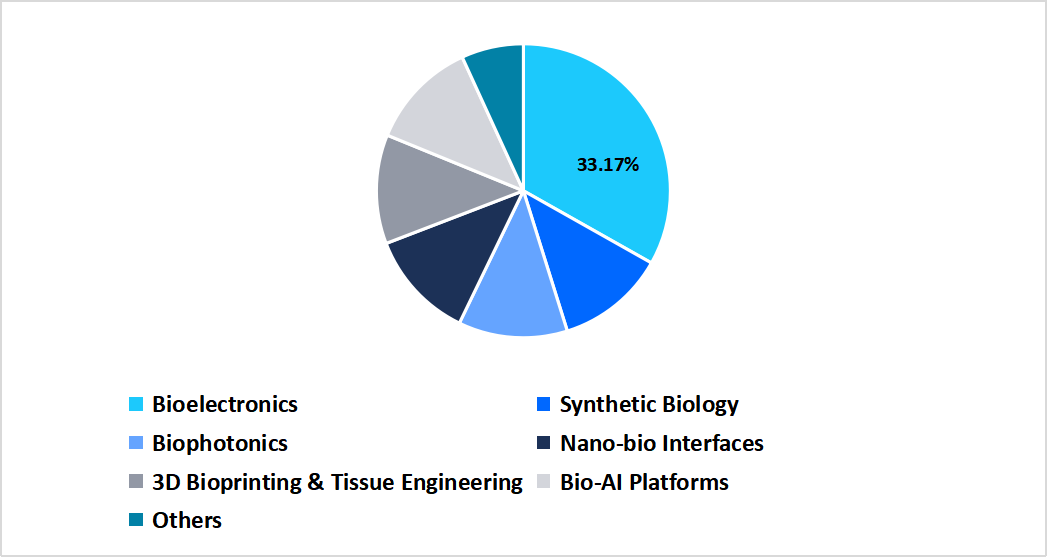
- Based on convergence technology, the bioelectronics segment dominated the market, accounting for a revenue share of 33.17% in 2025.
- On the basis of application, the drug discovery & development segment is anticipated to register the fastest CAGR of 8.43%.
- The US dominates the market, valued at USD 59.89 billion in 2024 and reaching USD 63.87 billion in 2025.
Table: U.S. Bioconvergence Technology Market Size (USD Million)
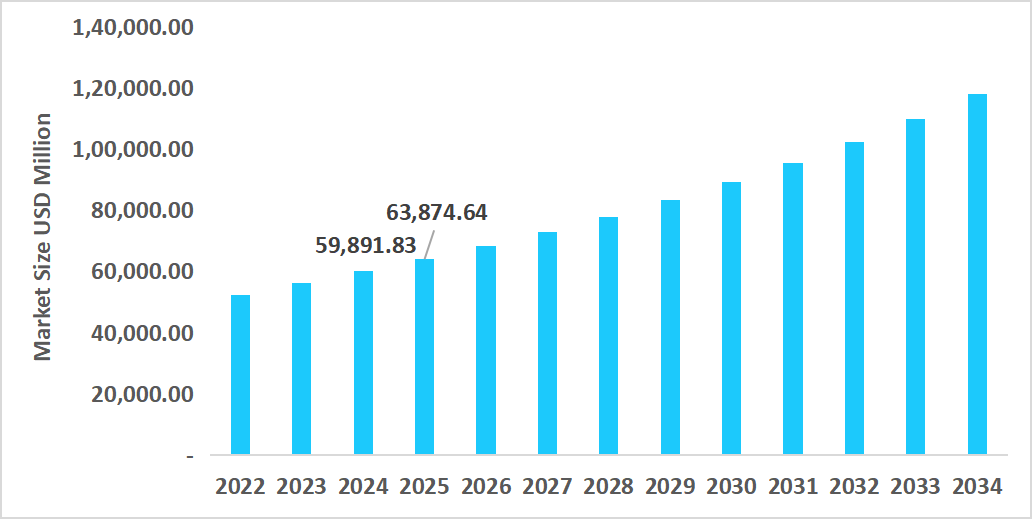
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 152.46 billion
- 2034 Projected Market Size: USD 281.2 billion
- CAGR (2026-2034): 7.08%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The global bioconvergence technologies market spans integrated platforms combining biology, engineering, and data science to advance research, diagnostics, and care delivery. Technology coverage includes bioelectronics, synthetic biology, biophotonics, nano bio interfaces, 3D bioprinting and tissue engineering, bio AI platforms, and others. Applications include diagnostics and imaging, drug discovery and development, precision and personalized medicine, regenerative medicine and tissue engineering, healthcare analytics and decision support, and others across clinical settings.
Latest Market Trends
Shift from Lab Research to AI-Driven Drug Discovery
Drug discovery in the biopharma sector is moving from conventional trial and error laboratory experimentation to AI-powered platforms that accelerate the identification and development of new therapeutics. Traditionally, researchers relied on iterative lab experiments, which were time-consuming, costly, and often inefficient in predicting drug performance. Today, AI-driven platforms offer predictive modeling, virtual screening, and simulation tools that enable faster identification of promising drug candidates. Platforms such as Iambic Therapeutics’ “Enchant” demonstrated how AI models forecast early-stage drug performance with higher accuracy than traditional approaches.
This AI-enabled approach reduced development costs, shortened timelines, and supports rapid innovation, marking a significant shift toward more efficient and technology-centric drug discovery in the bioconvergence technology market.
Growing Use of 3D Bioprinting in Regenerative Medicine
The adoption of 3D bioprinting technologies is emerging as a key trend in the bioconvergence technology market. In the past, tissue engineering and regenerative medicine faced limitations due to reliance on conventional lab-based cell cultures and scaffolding methods. Currently, 3D bioprinting enables the fabrication of complex, functional tissues with high reproducibility, supporting both drug testing and potential transplantation applications. For instance, in March 2024, researchers at the University of Virginia successfully 3D bioprinted functional liver tissue, demonstrating the feasibility of creating tissue models for pharmaceutical testing and regenerative therapies.
Hence, growing adoption of 3D bioprinting accelerated innovation in regenerative medicine, facilitated personalized therapies, and strengthened the development of bioconvergence technologies.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 152.46 Billion |
| Estimated 2026 Value | USD 162.97 Billion |
| Projected 2034 Value | USD 281.20 Billion |
| CAGR (2026-2034) | 7.08% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | BiomX, Singota Solutions, Anima Biotech Inc., Ginkgo Bioworks, SetPoint Medical |
to learn more about this report Download Free Sample Report
Market Drivers
Growing Approvals and Expanded Use of Cell & Gene Therapies
Recent regulatory approvals and label expansions of advanced biologic therapies are driving the bioconvergence technology market, as more treatments combining biology, engineering, and genetics become available. For instance, in April 2025, the U.S. FDA approved Zevaskyn (pz-cel) developed by Abeona Therapeutics, the first cell-based gene therapy for recessive dystrophic epidermolysis bullosa (RDEB), a rare genetic skin disorder. Thus, increased approvals and broader indications for cell & gene therapies are a major factor fueling demand for bioconvergence platforms.
Rising Adoption of Precision Medicine Fuels the Demand for Bioconvergence Technologies
The growing focus on personalized and precision medicine is driving demand for bioconvergence technologies, as it combines biology, engineering, and AI to create targeted therapies. The U.S. National Cancer Institute reported that over 45% of new oncology treatments in clinical trials involved precision medicine approaches, including gene and cell-based therapies.
Thus, the growing emphasis on personalized treatments is driving the adoption of bioconvergence platforms and supporting market growth.
Market Restraints
Shortage of Skilled Workforce and Technical Expertise
The adoption of bioconvergence technologies is limited by the scarcity of trained professionals in the intersection of biology and computational sciences technologies. The U.S. biotech firms reported that nearly 40% of advanced therapy projects faced delays due to a lack of skilled personnel capable of handling bioinformatics and lab-on-a-chip technologies.
Thus, the shortage of qualified personnel remains a significant barrier to the growth of the bioconvergence technology market.
Market Opportunity
Application of Organ-on-Chip Models in Drug Testing
Growing pressure to cut animal use in drug discovery and improve the prediction of human responses is pushing drug developers toward organ-on-chip models, a part of bioconvergence technology. High throughput platforms now let labs culture, maintain, and image many chips at once in a single, closed workstation, streamlining experiments and data capture. Emulate Inc.’s AVA Emulation System, a self-contained organ-on-a-chip workstation that cultures, incubates, and images up to 96 independent chips in parallel, shows how vendor innovation is lowering adoption barriers for pharmaceutical companies and CROs. As these platforms enable faster, more reproducible studies and richer mechanistic readouts, they create clear openings for bioconvergence players across instruments, reagents, software analytics, and contract testing services.
Regional Analysis
North America dominated the market in 2025, accounting for 44.73% market share. This dominance is attributed to the presence of leading pharmaceutical and biotech companies in North America, which fosters a competitive environment, drives innovation, and accelerates the development of bioconvergence solutions. In 2025, U.S. pharmaceutical companies allocated about USD 145.5 billion to R&D, reflecting a 4.5% increase from the previous year. This significant investment supports advancements in personalized medicine, making North America a dominant region in the bioconvergence technology market.
The U.S. leads the bioconvergence technology market due to the growing collaborations between technology providers and pharmaceutical companies also drive the demand for bioconvergence technologies. Merck and IMEC announced a strategic partnership to develop an advanced microphysiological systems (MPS) platform. This collaboration integrates organoid biology models with advanced semiconductor hardware, incorporating biosensing and microfluidic capabilities. Thus, the use of high-tech innovation, rapid technology adoption, and industry-academic collaborations drives the market growth of the bioconvergence technology market in the U.S.
Asia Pacific Market Insights
Asis Pacific is emerging as the fastest-growing region with a CAGR of 9.38% from 2026-2034, owing to the rapid expansion of biotech startups in China, India, and South Korea. Additionally, cost advantages in clinical research are also a major factor boosting the market growth in this region.
Australia's bioconvergence technology market growth is driven by the government's strategic investment in clinical trial infrastructure. Australia allocated USD 18.8 million over two years to develop the National One Stop Shop for Clinical Trials and Human Research. The government is fostering a conducive environment for bioconvergence innovations, thereby contributing to the market's expansion and increasing its market size. However, the absence of a national bioeconomy strategy restrains market growth.
Europe Market Insights
Europe is witnessing steady expansion in the bioconvergence technology market. Considerable market growth is driven as governments and hospital networks prioritized interoperable health data and shared evaluation pathways. Common data standards and cross-border research groups make it easier to compare outcomes across sites, shorten evidence cycles, and align buying decisions. Vendors can run multicentre pilots once, then scale across countries without rebuilding integrations. This policy coordination lowers adoption friction for bioconvergence tools in diagnostics, analytics, and therapy support, turning fragmented demand into predictable orders for validated platforms.
The UK bioconvergence technology market growth is driven by strategic government initiatives that promote innovation and collaboration within the life sciences sector. For example, in October 2024, Eli Lilly partnered with UK authorities to establish an “innovation accelerator” that provides resources and fosters partnerships for early stage biotech companies. These programs enhance technology development and facilitate faster commercialization of advanced therapies, making UK a significant country in the bioconvergence technology market.
Middle East and Africa Market Insights
The MEA market advanced as governments expanded public private partnerships and multi hospital build programs. Large developers and health ministries bundle equipment, software, and multiyear service into one procurement, favoring integrated platforms that can be deployed quickly across new hospitals and clinics. Vendors that can finance, install, train, and support at scale secure network wide contracts. This commercial model reduces buying complexity for health systems and concentrates spend with suppliers of bioconvergence solutions.
The Saudi Arabian market is witnessing stable growth due to the high prevalence of diabetes, obesity, cardiovascular disease and hereditary disorders is raising demand for earlier detection and continuous management. As a result, providers are prioritizing bioconvergence solutions that unite advanced diagnostics, bioelectronic monitoring and AI supported care with streamlined lab workflows. These tools improve risk stratification, therapy adjustment and remote follow up at scale, accelerating adoption.
Latin America Market Insights
In Latin America, large private hospital and laboratory chains are centralizing purchasing. They pick one technology stack for many sites and sign region wide contracts with a few trusted vendors. This simplifies decisions, ensures consistent training and service, and lowers total costs. As a result, scalable bioconvergence platforms are deployed faster and more broadly across systems.
The Brazilian market is growing as government partnership programs link public procurement to local production and technology transfer. Suppliers that manufacture in country receive preference, gaining predictable orders and quicker certification. This policy builds reliable supply and service capacity, boosting uptake of bioconvergence platforms across Brazil’s public hospital and laboratory networks.
Regional Market share (%) in 2025
Source: Straits Research
Convergence Technology Insights
The bioelectronics segment dominated the market, accounting for a revenue share of 33.17% in 2025. This dominance is attributed to the increasing integration of bioelectronic devices in advanced healthcare applications and growing regulatory approvals for implantable and wearable devices. These devices enable modulation of physiological signals and improve treatment outcomes for chronic diseases such as diabetes, heart failure, and neurological diseases. Thus, all aforementioned factors support segmental market growth.
The synthetic biology segment is anticipated to witness the fastest growth rate of 8.1% during the forecast timeframe. Substantial growth of the segment is majorly due to the shift by consumer and specialty chemical brands toward bio-made ingredients to meet sustainability goals and retailer standards, which is driving multi-year purchase agreements. These commitments secure demand, unlock funding for bigger fermentation capacity, lower production costs, and speed product launches across enzymes, materials, flavors, and fragrances.
By Convergence Technology Market Share (%), 2025
Source: Straits Research
Application Insights
The drug discovery & development segment is anticipated to register the fastest CAGR of 8.43% during 2026-2034. This growth is attributed to the increasing use of bioconvergence technologies such as lab-on-a-chip platforms and high-throughput organ-on-chip systems for faster and accurate target identification in drug discovery. Additionally, the use of computational biology with cell and gene therapies reduced trial and error in preclinical testing. These advantages offered by bioconvergence technologies have increased the collaborations between biotech and pharma companies to accelerate drug development, making it the fastest-growing segment.
The diagnostics and imaging segment dominated the market, capturing 29.8% of revenue share in 2025. The segment led because accreditation requirements such as ISO 15189, CAP, and CLIA emphasize consistent results, documented quality controls and traceable workflows, which are well supported by integrated bioconvergence platforms. Bioconvergence diagnostics bring sample preparation, imaging, and automated QC into one system, generating audit-ready records by default. That makes passing inspections easier and reduces retests. Labs that meet standards keep reimbursement and win tenders, so they prioritize buying and upgrading these platforms. As more labs adopt them, spending concentrates here, driving the diagnostics and imaging segment.
Competitive Landscape
The global bioconvergence technology market is moderately consolidated, with several key players holding significant market share. The major players in the market include BiomX, Singota Solutions, Anima Biotech Inc., Ginkgo Bioworks, SetPoint Medical, Galvani Bioelectronics, and others.
The industry players are inclined to adopt various strategic initiatives such as mergers and acquisitions, collaborations with biotech startups, and investing in next-generation drug discovery to stay competitive in the market.
Barcode Nanotech: An emerging market player
Barcode Nanotech is an innovative company specializing in the development of target-specific nanoparticles for next-generation RNA therapies. Their focus is on enhancing the precision and efficacy of RNA-based treatments.
- In 2024, Barcode Nanotech secured USD 6.1 million in seed funding to advance its research and development efforts in RNA-based therapies.
List of Key and Emerging Players in Bioconvergence Technology Market
- BiomX
- Singota Solutions
- Anima Biotech Inc.
- Ginkgo Bioworks
- SetPoint Medical
- Galvani Bioelectronics
- BICO - The Bio Convergence Company
- Thermo Fisher Scientific Inc.
- Cytena
- Century Therapeutics
- BioConvergent Health
- Pangea Biomed
- Cellular Engineering Technologies, Inc.
- Merck Co., Inc.
- Synkriom Digital
- Biodesign Israel
- Ezassi, Inc.
- 3D Systems, Inc.
- Regemat 3D
- Materialise
- Others
Strategic Initiatives
- 11th June 2025: NVIDIA has partnered with Novo Nordisk and the Danish Centre for AI Innovation (DCAI) to advance drug discovery through the integration of AI technologies. This collaboration was focused on utilizing NVIDIA's AI platforms, including BioNeMo, NeMo, NIM, and Omniverse, for accelerating the development of pharmaceutical R&D.
- In January 2024, AION Labs launched TenAces Biosciences to apply AI and biology for molecular glue drug discovery, advancing the bioconvergence of computational and therapeutic sciences.
- In October 2025, Takeda partnered with Nabla Bio to apply generative AI for protein and antibody drug design, advancing bioconvergence between computational modeling and biologic therapeutics development
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 152.46 Billion |
| Market Size in 2026 | USD 162.97 Billion |
| Market Size in 2034 | USD 281.20 Billion |
| CAGR | 7.08% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Convergence Technology, By Application |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Bioconvergence Technology Market Segments
By Convergence Technology
- Bioelectronics
- Synthetic Biology
- Biophotonics
- Nano-bio Interfaces
- 3D Bioprinting & Tissue Engineering
- Bio-AI Platforms
- Others
By Application
- Diagnostics & Imaging
- Drug Discovery & Development
- Precision & Personalized Medicine
- Regenerative Medicine & Tissue Engineering
- Healthcare Analytics & Decision Support
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































