Cell and Gene Therapy Manufacturing QC Market Size, Share & Trends Analysis Report By Therapy Type (Cell Therapy [CAR-T {Autologous CAR-T, Allogeneic CAR-T}, CAR-NK, B-Cell, & Others], and Gene Therapy [Viral {AAV, Lenti, and Other}, Non-Viral]), By Scale (Pre-commercial/ R&D Manufacturing, Commercial Scale Manufacturing), By Mode (Contract Manufacturing, In-house Manufacturing), By Workflow (Cell Processing, Cell Banking, Fill & Finish Operations, Raw Material Testing, Vector Production, Other Workflows), By Process (Upstream Processes, Downstream Processes), By End-User (Pharmaceutical Companies, Biopharmaceutical / Biotechnological Companies, Contract Manufacturing Organizations), By Technology Type (Fluorescence-Activated Cell Sorting (FACS), Enzyme-Linked Immunosorbent Assay (ELISA), Chromatography, Next-Generation Sequencing (NGS), Polymerase Chain Reaction (PCR), Others) and By Region (North America, Europe, Asia Pacific, Middle East & Africa, Latin America) Forecasts, 2025-2033
Cell and Gene Therapy Manufacturing Qc Market Size
The global cell and gene therapy manufacturing QC market size was valued at USD 2.24 billion in 2024 and is projected to grow from USD 2.82 billion in 2025 to USD 17.55 billion by 2033, exhibiting a CAGR of 25.7% during the forecast period (2025-2033).
The major factors driving the market are growing pipeline of cell & gene therapy (CGT), increasing investments in advanced therapy medicinal products (ATMPs) sector including cell & gene therapy, and increasing number of CAR-T cell therapy trials. Further, regulatory requirements for CGT products drive the demand robust QC services to ensure safety and efficacy of CGT therapies. Furthermore, the growing number of therapeutic developers is also propelling the adoption of outsourced QC services, automation, and AI-driven analytics, streamlining compliance and improving efficiency.
Moreover, investments in next-generation sequencing (NGS), multi-attribute methods (MAM), and biosafety testing are further shaping the future of QC in this sector. As the industry scales up production to meet rising global demand, the market will continue to expand, ensuring the safety, efficacy, and regulatory compliance of innovative therapies. The graph illustrates the distribution of gene therapy, GMCT & CBIO, and cell therapy developers across North America, Europe, Asia-Pacific, and other regions. It highlights North America's dominance in gene therapy and Europe’s strong presence in GMCT & CBIO and cell therapy development.
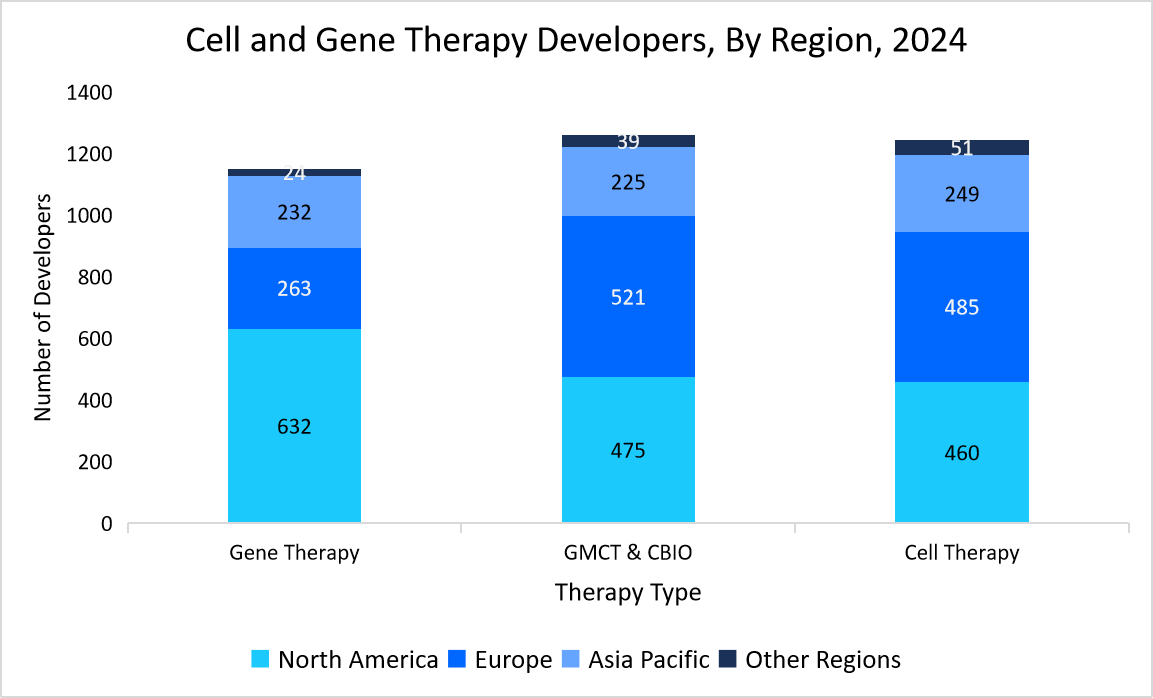
Source: Alliance for Regenerative Medicine, and Straits Research
Cell and Gene Therapy Manufacturing Qc Market Trends
Outsourcing Qc Activities: Optimizing Efficiency in Cgt Manufacturing
As CGT manufacturers focus on accelerating production and commercialization, outsourcing QC activities to specialized contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) is becoming a key trend.
- For example, the below graph illustrates the adoption of QC outsourcing by UK cell and gene therapy companies.
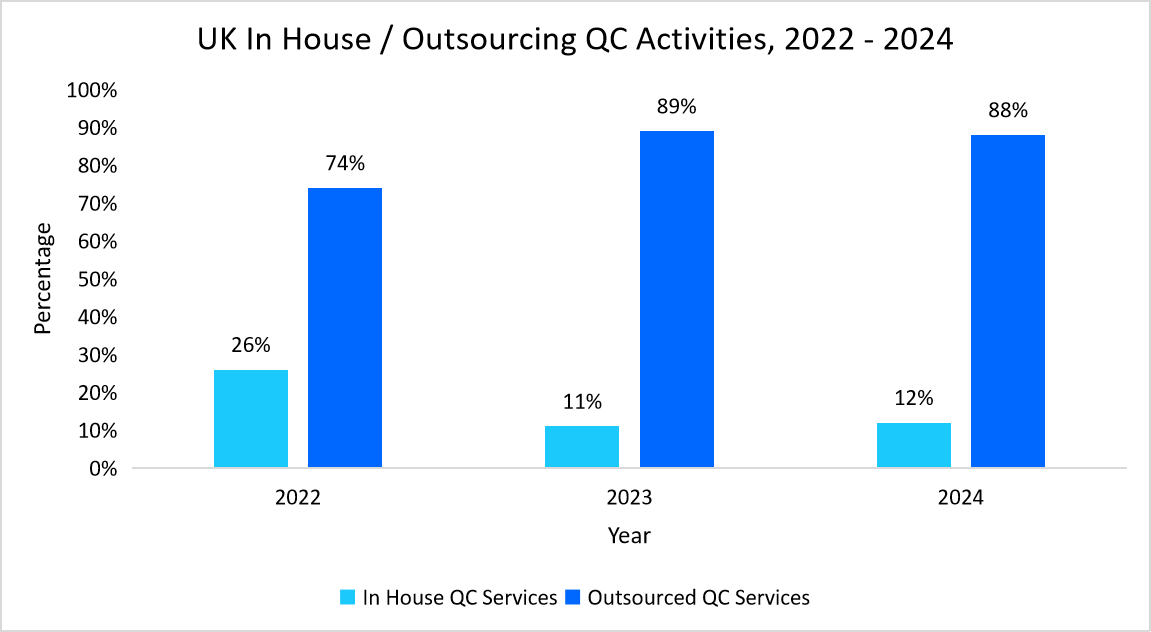
Source: Cell and Gene Therapy Catapult Review Reports, and Straits Research
Thus, this trend reflects a strategic shift towards external partnerships to enhance efficiency, ensure compliance, and streamline CGT manufacturing.
Ai-Powered Real-Time Analytics: Transforming Qc in Cgt Manufacturing
The integration of real-time analytics and AI-driven insights is transforming quality control in cell and gene therapy manufacturing by enhancing decision-making, improving process efficiency, and ensuring regulatory compliance.
- For example, TetraScience's Scientific Data and AI Cloud integrates real-time data from instruments and systems, allowing QC teams to apply AI-driven insights for faster and more reliable decision-making.
Thus, this trend is driving a shift from reactive to proactive QC strategies, improving product safety and accelerating time-to-market.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 2.24 Billion |
| Estimated 2025 Value | USD 2.82 Billion |
| Projected 2033 Value | USD 17.55 Billion |
| CAGR (2025-2033) | 25.7% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | WuXi AppTec, Lonza, Thermo Fisher Scientific, Charles River Laboratories, Catalent |

to learn more about this report Download Free Sample Report
Cell and Gene Therapy Manufacturing Qc Market Drivers
Surging Investments Accelerating Qc Advancements in Cgt Manufacturing
Rising investments in cell and gene therapy manufacturing are driving advancements in QC infrastructure, technologies, and workforce development to meet regulatory and industry demands.
- For example, the following graph highlights the increasing investments (from 2022 to 2024) in regenerative medicine, including cell and gene therapy, underscoring the growing demand for QC services.
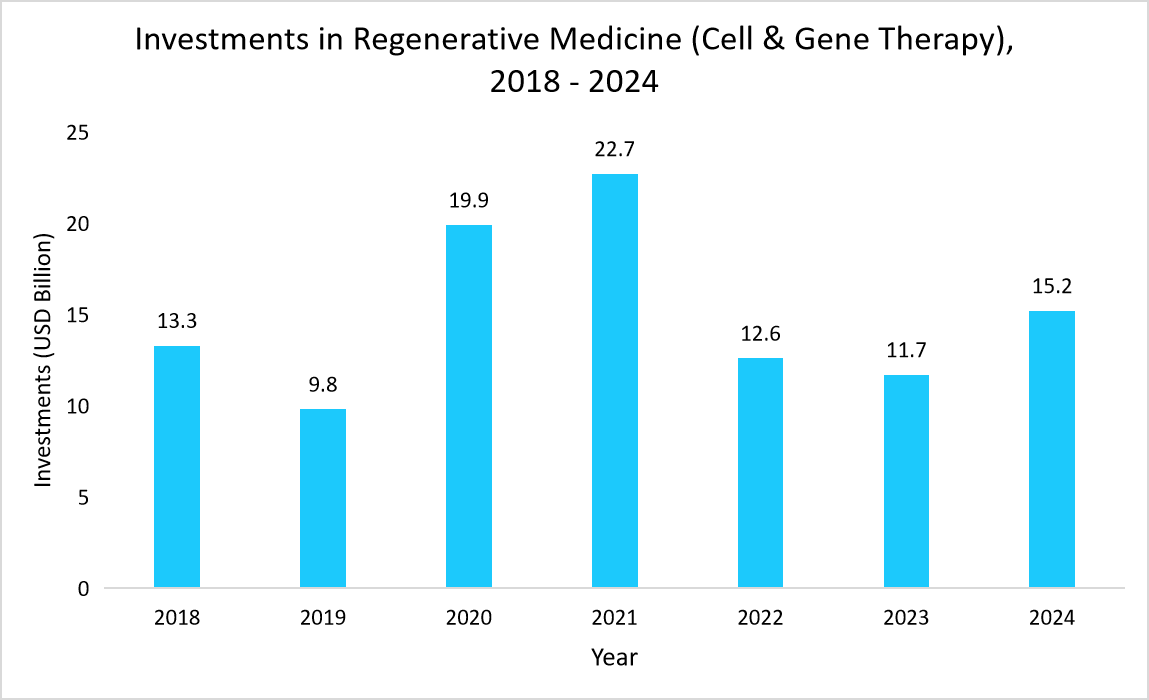
Source: Alliance for Regenerative Medicine, and Straits Research
Thus, growing investments in CGT manufacturing are accelerating advancements in QC infrastructure, ensuring robust quality standards, regulatory compliance, and scalable production.
Regulatory Requirements for Cgt Manufacturing
Stringent regulatory requirements drive the growth of the cell and gene therapy manufacturing QC sector by ensuring strict compliance and safety standards. Advanced therapeutic products are highly complex and represent health risks and safety concerns to patients, which is why they require strict control and compliance for therapeutic use. Regulatory authorities worldwide, including the FDA and the EMA, have developed comprehensive frameworks and guidelines that regulate cell and gene therapy development, production, and commercialization.
- For example, in January 2024, the Center for Biologics Evaluation and Research, FDA issued guidelines for Gene Therapy Products and Development of Chimeric Antigen Receptor (CAR) T Cell Products.
- Similarly, EMA’s joint clinical assessments and stricter guidelines starting in 2025, drive the demand for standardized and validated potency and identity assays.
Thus, regulatory policies and stricter guidelines are accelerating the demand for robust QC measures, driving growth in the cell and gene therapy manufacturing industry.
Market Restraining Factors
Expanding Skills Gap and Labor Shortage
The expanding skills gap and labor shortage is the major factor restraining the growth of the market. As industry continues to evolve rapidly, the demand for highly skilled and experienced QC professionals is outpacing the supply, creating a critical workforce challenge, thereby restraining the market's growth.
- For example, an article published by Charles River Laboratories in May 2023 depicted that the number of CAR-T job advertisements by three top manufacturers doubled between 2019 and 2020 from 123 to 203 despite the ongoing COVID-19 pandemic.
- Further, as per the Alliance for Regenerative Medicine report, in March 2023, the U.S. Cell and Gene Therapy Sector will hire more than 32,000 workers in commercialization and R&D by 2025 to fill the labor shortage.
Thus, the skills gap and labor shortage pose a significant challenge, limiting the market's growth and scalability.
Market Opportunity
Growing Pipeline of Cell and Gene Therapy Products
The rapidly growing pipeline of CGT products presents a significant opportunity for the market, driving the demand for advanced testing solutions to ensure compliance, safety, and efficacy. As more and more cell and gene therapy candidates advance through the development stages and move closer to commercialization, the demand for comprehensive QC testing and validation increases substantially, propelling the demand for QC services.
- For example, the table below shows several ongoing clinical studies of gene therapy for the 2023 – 2024 period. The table depicts that the number of gene therapies increased from 2,093 (Q1, 2024) to 2,117 (Q4, 2024), thereby resulting in a demand for QC technologies.
Gene Therapy Pipeline, 2024
| Global | Q1, 2024 | Q2, 2024 | Q3, 2024 | Q4, 2024 |
| Preclinical | 1,471 | 1,436 | 1,393 | 1,424 |
| Phase I | 301 | 314 | 318 | 341 |
| Phase II | 282 | 279 | 289 | 306 |
| Phase III | 35 | 34 | 35 | 35 |
| Preregistration | 4 | 5 | 6 | 11 |
| Total | 2,093 | 2,068 | 2,041 | 2,117 |
Source: American Society of Gene & Cell Therapy (ASGCT), and Straits Research
Thus, the expanding CGT pipeline is driving the need for advanced QC testing to ensure regulatory compliance, safety, and efficacy.
Regional Insights
North America: Dominant Region with 38.6% Market Share
North America holds a leading position in the cell and gene therapy manufacturing QC market owing to strong regulatory frameworks (FDA guidelines), high R&D investments, advanced QC infrastructure, and a robust pipeline of CGT products. The presence of key industry players, extensive clinical trials, and government funding initiatives further drives market growth, ensuring compliance and innovation in QC processes.
Asia Pacific: Fastest Growing Region with the Highest Market Cagr
Asia Pacific region is anticipated to grow at fastest CAGR of 26.3% during the forecast period. This is attributed to governments and private entities in countries such as China, Japan, and South Korea actively investing in biotech infrastructure and accelerating the adoption of cell and gene therapy. Furthermore, growing demand for advanced therapies, expansion of contract development, and manufacturing organizations are driving the need for QC services in this region.
Countries Insights
The cell and gene therapy manufacturing QC sector is experiencing dynamic growth and diversification across the globe, with each county contributing uniquely to the advancement of this transformative field.
- U.S.- U.S. dominates the cell and gene therapy manufacturing QC industry, driven by strong FDA regulations, extensive clinical trial activity, and heavy investments in advanced QC technologies. The presence of leading CGT manufacturers, specialized QC service providers, and academic research institutions fosters continuous innovation. Additionally, initiatives such as the Biden Administration’s ARPA-H and the NIH’s regenerative medicine programs are accelerating QC advancements and commercialization efforts.
- Canada– Canada’s market is expanding owing to strong innovation, strategic partnerships, and government support. Collaborations such as Catamaran Bio and OmniaBio’s CAR-NK therapy development and USD 59 million in funding for biotherapeutics at Ottawa Hospital are driving demand for QC services. Additionally, rising regulatory approvals, such as Health Canada’s approval of BMS’s Breyanzi for DLBCL in January 2025, highlight the growing need for stringent QC measures in CGT manufacturing.
- Germany- Germany’s sector is growing rapidly, driven by a thriving biotech ecosystem, active R&D, and a high volume of CGT clinical studies. Additionally, Germany is formulating new national strategies to enhance CGT manufacturing and adoption, further boosting the demand for QC services. The graph below illustrates the growing number of CGT clinical trials, highlighting the increasing need for stringent QC measures.
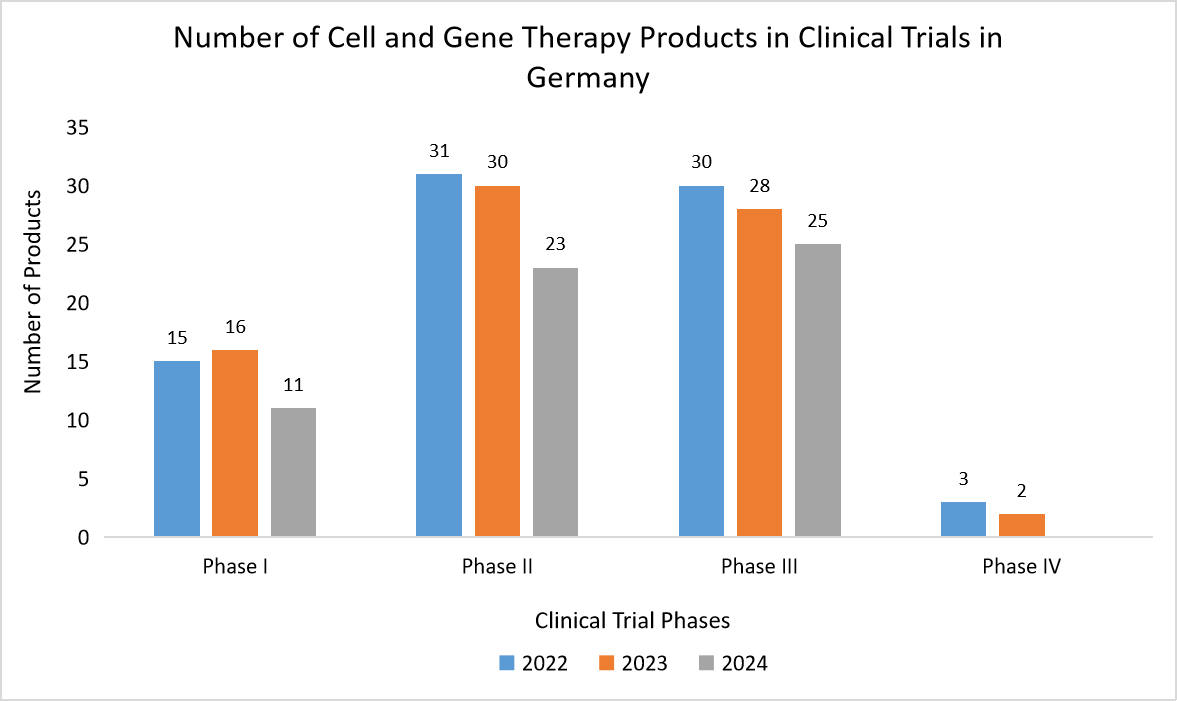
Source: Clinical Trials.gov, and Straits Research
- France - France’s market is driven by strong government support, strategic investments, and pioneering research initiatives. Programs such as "Plan France Médecine Génomique 2025" and the USD 8.8 billion Healthcare Innovation Plan 2030 fuel CGT advancements, with USD 1.18 billion dedicated to biomedical research. As innovation accelerates and multiple therapies progress through development, the demand for robust QC services continues to rise.
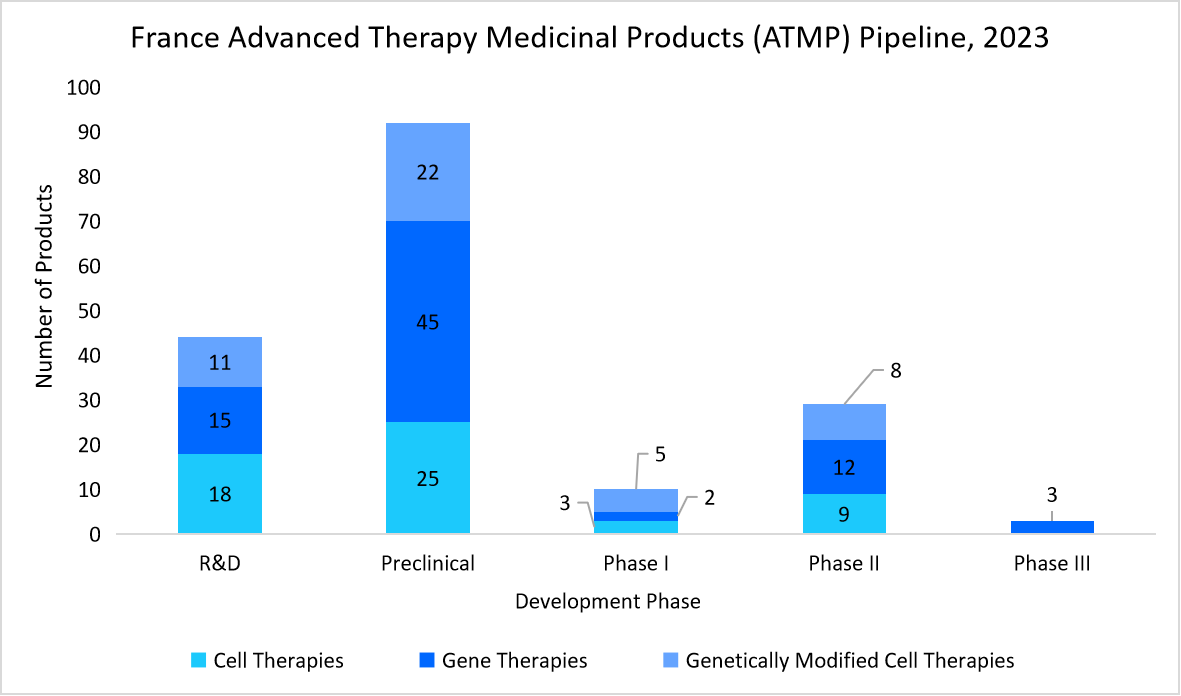
Source: Health Innovation Agency, and Straits Research
- UK – The major factors driving the growth of UK market are growing investments in CGT sector, expanding GMP manufacturing facility, and increasing number of approvals. For instance, the below graph shows rise in facility space from 20,500 m² in 2020 to 51,862 m²in 2024 reflects the industry's need for larger-scale production, indicating growing investment in ATMP manufacturing including cell and gene therapy segment.
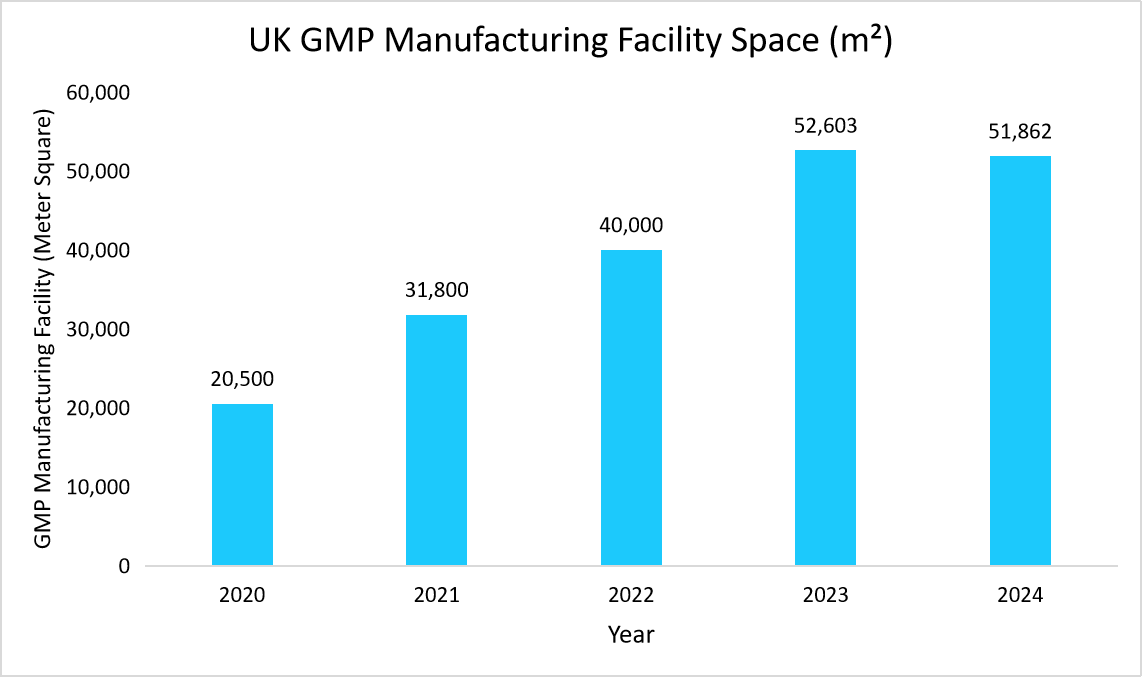
Source: Cell and Gene Therapy Catapult Review Reports, and Straits Research
- China - China’s market is expanding rapidly, driven by a surge in clinical trials and a growing CGT pipeline. According to the National Center for Biotechnology Information, in February 2023, CGT pipelines grew by 17% from the year 2010 to 2022. China’s focus on CAR-T cell therapy and rising regulatory approvals are further propelling the demand for QC services during scale-up manufacturing. Among the 2,034-cell therapy-related trials, 1,164 are dedicated to CAR-T therapies, with 11 products in the pivotal stage and two filed under New Drug Applications (NDAs) with the NMPA. This rapid progress underscores the growing need for robust QC measures to support commercialization.
Therapy Type Analysis
The market is segmented into cell therapy [CAR-T {Autologous CAR-T, Allogeneic CAR-T}, CAR-NK, B-Cell, & Others] and gene therapy [Viral {AAV, Lenti, and Other}, Non-Viral]. Gene therapy segment is expected to register fastest CAGR during the forecast period, owing to increasing number of FDA and EMA approvals for gene therapies, growing pipeline of gene therapy, and strict guidelines for purity and potency of gene therapies are driving the need for robust QC services.
Scale Analysis
The market is segmented into pre-commercial/ R&D manufacturing and commercial scale manufacturing. Pre commercial/ R&D Manufacturing segment dominated the market in 2024 and is expected to register a highest CAGR during the forecast period. This growth is driven by, increasing number of cell and gene therapy products under clinical trials, autologous and allogeneic cell therapy platforms, and a high failure rate in late-stage clinical trials has increased the activity in the pre-commercial phase.
Mode Analysis
The market is bifurcated into contract manufacturing and in-house manufacturing. Contract manufacturing segment is expected to register fastest CAGR during the forecast period, owing to surge in outsourcing of QC processes to CDMOs which are equipped with advanced analytical technologies and compliant with regulatory standards.
Workflow Insights
The market is segmented into cell processing, cell banking, process development, fill & finish operations, raw material testing, vector production, and other workflows. Vector production segment accounted for largest share in 2024, owing to increasing demand for gene replacement therapies, drives the need for ensuring quality and efficiency of vectors, which in turn, drives the demand for vector production QC service.
Process Analysis
The market is segmented into upstream processes and downstream processes. Downstream processes segment is expected to register fastest CAGR of 25.3% during the forecast period, owing to advancement in bioprocessing technologies that has improved the downstream processing, rise in demand for high purity CGT products and expansion of contract manufacturing organizations are collectively driving the segment growth.
End User Insights
The market is segmented into, pharmaceutical companies, biopharmaceutical/biotechnological companies, and contract manufacturing organizations. Biopharmaceutical / Biotechnological Companies dominated the market in 2024, as major pharmaceutical companies such as Novartis and Gilead Sciences have increased their pipeline candidates with cell and gene therapies and growing collaborations between biopharma companies further drives the segment growth.
Technology Insights
The market is segmented into fluorescence-activated cell sorting (FACS), enzyme-linked immunosorbent assay (ELISA), chromatography, next-generation sequencing (NGS), polymerase chain reaction (PCR), and others. Polymerase chain reaction (PCR) dominated the market owing to its high sensitivity, rapid turnaround, and regulatory compliance in detecting genetic modifications and contaminants. The adoption of qPCR and dPCR for precise quantification and the rising need for standardized potency and identity assays further contributes to the segment growth.
Company Market Share
Key players in the industry are focus on adopting key business strategies, such as strategic collaborations, acquisitions, and expansions, to gain a strong foothold in the market.
Solvias: An Emerging Player in the Cell and Gene Therapy Manufacturing Qc Market
Solvias is an emerging player in the market, specialized in method development, validation, stability testing, and regulatory compliance support for CGT.
Recent Developments by Solvias:
- In January 2025, Solvias, a global leader announced the opening of its Center of Excellence for Biologics and Cell & Gene Therapy in the heart of Research Triangle Park (RTP), North Carolina.
List of Key and Emerging Players in Cell and Gene Therapy Manufacturing QC Market
- WuXi AppTec
- Lonza
- Thermo Fisher Scientific
- Charles River Laboratories
- Catalent
- Merck KGaA
- Eurofins Scientific
- Pace
- Pharmaron
- BioAgilytix Labs
- Avance Biosciences
- SGS
- IQVIA
- Alcami Corporation
- Solvias
- Pacific BioLabs
- ProtaGene
- Laboratory Corporation of America Holdings
- AGC Biologics
- uBriGene Biosciences International Co.
Recent Developments
- In March 2025, Alfresa Holdings Corporation and Minaris Regenerative Medicine Co., Ltd. signed a Memorandum of Understanding (MoU) to collaborate on cell therapy development and manufacturing, aiming to support clinical and commercial production of cell therapy products in Japan.
- In January 2025, Catalent, Inc. partnered with Galapagos NV to support decentralized manufacturing for clinical studies of GLPG5101, an investigational CAR-T therapy for relapsed/refractory NHL.
Analyst Opinion
The cell and gene therapy manufacturing QC market is witnessing significant growth, driven by stringent regulatory requirements for CGT manufacturing, increasing adoption of automated and AI-driven QC solutions, a growing pipeline of CGT products, and rising investments in advanced QC infrastructure. Additionally, the outsourcing of QC activities to specialized CROs and CDMOs is streamlining compliance and scalability, while real-time analytics and predictive quality control are enhancing manufacturing efficiency. Expanding global collaborations and government funding initiatives further accelerate the market by supporting innovation and standardization in QC processes.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 2.24 Billion |
| Market Size in 2025 | USD 2.82 Billion |
| Market Size in 2033 | USD 17.55 Billion |
| CAGR | 25.7% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Therapy Type, By Scale, By Mode, By Workflow, By Process, By End-User, By Technology Type |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Cell and Gene Therapy Manufacturing QC Market Segments
By Therapy Type
-
Cell Therapy
-
CAR-T
- Autologous CAR-T
- Allogeneic CAR-T
- CAR-NK
- B-Cell
- Others
-
CAR-T
-
Gene Therapy
-
Viral
- AAV
- Lentiviral vectors
- Other
- Non-Viral
-
Viral
By Scale
- Pre-commercial/ R&D Manufacturing
- Commercial Scale Manufacturing
By Mode
- Contract Manufacturing
- In-house Manufacturing
By Workflow
- Cell Processing
- Cell Banking
- Fill & Finish Operations
- Raw Material Testing
- Vector Production
- Other Workflows
By Process
- Upstream Processes
- Downstream Processes
By End-User
-
Pharmaceutical Companies
- Small Size
- Medium Size
- Large Size
-
Biopharmaceutical / Biotechnological Companies
- Small Size
- Medium Size
- Large Size
-
Contract Manufacturing Organizations
- Small Size
- Medium Size
- Large Size
By Technology Type
- Fluorescence-Activated Cell Sorting (FACS)
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Chromatography
- Next-Generation Sequencing (NGS)
- Polymerase Chain Reaction (PCR)
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































