Cell Cryopreservation Market Size, Share & Trends Analysis Report By Product Type (Cryoprotectant Agents, Equipment, Others), By Application (Stem Cells, Oocytes and Embryotic cells, Sperm cells, Hepatocytes, Others), By End User (Biopharmaceutical & Pharmaceutical Companies, Research Institutes, Biobanks, IVF clinics, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Cell Cryopreservation Market Overview
The global cell cryopreservation market size is estimated at USD 3.30 billion in 2025 and is projected to reach USD 8.68 billion by 2034, growing at a CAGR of 11.37% during the forecast period. Sustained growth of the market is propelled by the increasing integration of cryopreservation technologies in advanced clinical research and large scale cell manufacturing processes.
Key Market Trends & Insights
- The North America held a dominant share of the global market, accounting for 44.17%.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 13.48%.
- Type: the equipment segment is anticipated to register the fastest CAGR of 12.32% during the forecast period.
- Application: The stem cells segment dominated the market in 2025, with a revenue share of 33.24%.
- End-User: The biopharmaceutical & pharmaceutical companies segment dominated the market in 2025, with a revenue share of 41.23%.
- The U.S. dominates the global market, valued at USD 1.19 billion in 2024 and reaching USD 1.32 billion in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
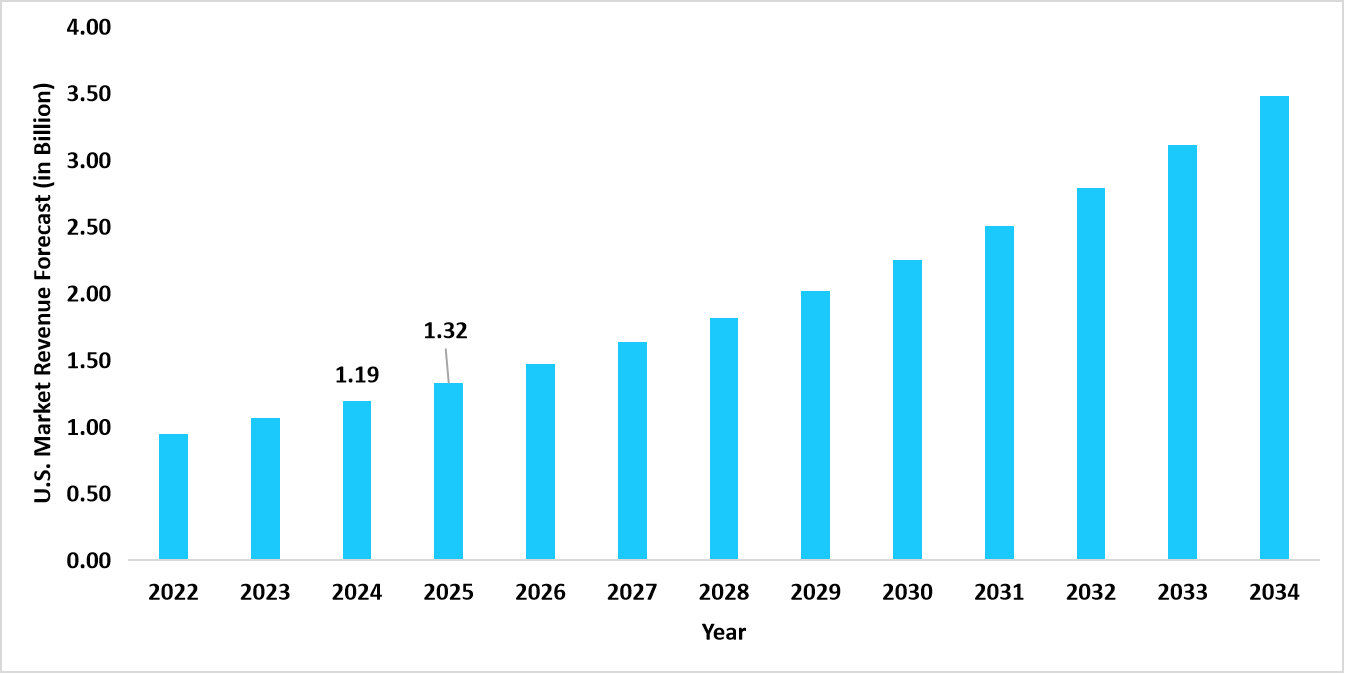
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 3.30 billion
- 2034 Projected Market Size: USD 8.68 billion
- CAGR (2025 to 2034): 11.37%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The cell cryopreservation market refers to the global industry focused on preserving living cells at ultra-low temperatures to maintain their structural integrity, functionality, and viability for future use in research and clinical applications. It comprises two key product categories: cryoprotectant agents such as glycerol, dimethyl sulfoxide (DMSO), and other protective formulations that prevent ice crystal formation and cellular damage, and equipment, including incubators, liquid nitrogen supply tanks, freezers, and other storage systems that ensure controlled temperature conditions for long-term preservation. Based on application, cell cryopreservation is widely used for the storage of stem cells, oocytes, and embryonic cells, sperm cells, hepatocytes, and other cell types critical to regenerative medicine, reproductive biology, and disease modeling. The market serves multiple end users such as biopharmaceutical and pharmaceutical companies, research institutes, biobanks, and IVF clinics, each leveraging cryopreservation technologies to support drug discovery, biologics manufacturing, cell therapy production, academic research, and reproductive treatments. Collectively, the cell cryopreservation market forms the technological backbone of regenerative medicine and cellular research, enabling the safe, efficient, and long-term preservation of valuable biological materials essential for innovation in modern healthcare.
Latest Market Trends
Transition Toward Automated and Closed System Cryopreservation Technologies
The cell cryopreservation market is experiencing a growing trend toward the adoption of automated and closed-system cryopreservation technologies that enhance process control, reproducibility, and contamination prevention. Traditional manual freezing methods are being replaced by fully automated cryogenic systems integrated with real-time monitoring and robotic handling to ensure consistent temperature regulation and standardized cell storage conditions. For instance, in February 2025, Cytiva launched its next-generation VIA Freeze system equipped with digital sensors and data logging capabilities to optimize cell freezing curves and improve viability outcomes. This shift toward automation and closed systems improved scalability, reduced human error, and supported compliance with good manufacturing practice (GMP) standards in cell therapy manufacturing and biobanking applications.
Rising Focus on Development of Novel Cryoprotectants and Ice-Free Preservation Methods
The emerging trend in the cell cryopreservation market is the development of novel cryoprotectant formulations and ice-free preservation techniques aimed at minimizing cellular damage during freezing and thawing. Conventional agents such as dimethyl sulfoxide (DMSO) are associated with cytotoxicity and post-thaw cell dysfunction, driving research toward non-toxic and biocompatible alternatives. In June 2025, researchers at the University of California developed a polymer-based cryoprotectant capable of preventing intracellular ice formation and maintaining over 90% cell viability after thawing. Additionally, advancements in vitrification and nano-engineered cooling systems are enabling faster cooling rates and improved structural integrity of preserved cells. This growing emphasis on safer, more efficient cryopreservation solutions is transforming long-term cell storage and expanding applications in regenerative medicine, stem cell banking, and reproductive biology.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 3.30 Billion |
| Estimated 2026 Value | USD 3.67 Billion |
| Projected 2034 Value | USD 8.68 Billion |
| CAGR (2026-2034) | 11.37% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Lonza, Thermo Fisher Scientific Inc., Merck KGaA, Sartorius AG, BioLife Solutions, Inc. |
Cell Cryopreservation Market Driver
Growing Adoption of Cell and Gene Therapies Driving Market Growth
A major driver for the cell cryopreservation market is the increasing adoption of cell and gene therapies across clinical and commercial applications. The rising number of FDA and EMA approvals for stem cell based and CAR T cell therapies has created strong demand for cryopreservation systems capable of maintaining cell integrity, viability, and potency throughout storage and transport. For instance, in 2025, the growing use of cryopreserved CAR T cell products in oncology treatments highlighted the critical role of advanced freezing and thawing solutions in ensuring consistent therapeutic outcomes. The integration of automated cryogenic storage platforms, improved cryoprotectant formulations, and digital monitoring technologies has further enhanced process efficiency and quality assurance in biobanking and cell therapy manufacturing. This expanding utilization of cryogenic preservation technologies across regenerative medicine and clinical research is driving the sustained growth of the global cell cryopreservation market.
Market Restraint
Technical Limitations and Cell Viability Loss During Thawing Restricting the Growth of the Cell Cryopreservation Market
A major restraint for the cell cryopreservation market is the loss of cell viability and functionality during the freezing and thawing processes. Despite advancements in cryoprotectants and controlled rate freezing systems, several cell types such as stem cells, hepatocytes, and primary immune cells remain highly sensitive to cryogenic stress, resulting in reduced recovery rates and altered biological performance post thaw. Inconsistent temperature maintenance during storage and transportation further exacerbates this challenge, particularly in large scale biobanking and clinical applications. Additionally, limited standardization in cryopreservation protocols across laboratories and manufacturing facilities complicates process validation and quality assurance. These technical limitations and reproducibility concerns hinder the efficient preservation of high value therapeutic cells, posing a major challenge to the broader adoption and scalability of cell cryopreservation technologies in research and clinical settings.
Market Opportunity
Integration of Artificial Intelligence and Automation Creating New Opportunities for the Cell Cryopreservation Markets
An emerging opportunity for the cell cryopreservation market lies in the integration of artificial intelligence (AI), automation, and digital monitoring technologies to enhance the efficiency, accuracy, and reliability of preservation processes. AI-enabled cryogenic systems are increasingly being developed to optimize temperature control, predict equipment failures, and maintain consistent cell viability through real time analytics and predictive maintenance. Automated biobanking solutions are also reducing human error and improving reproducibility in large scale cell storage operations. Moreover, expanding research and healthcare infrastructure in emerging economies, coupled with growing investments in biotechnology and life sciences, is creating major potential for global players to introduce cost efficient and technologically advanced cryopreservation solutions. As AI-driven automation becomes a key enabler of efficiency and quality assurance, it is expected to unlock new growth avenues for manufacturers and service providers in the global market.
Regional Analysis
North America region dominated the market in 2025, with a revenue share 44.17%. The driving factor for the North America Cell Cryopreservation market is the growing integration of automated cryogenic storage systems and controlled-rate freezers within biobanks and cell therapy manufacturing facilities. These automated platforms enhance process consistency, minimize human error, and ensure long-term cell viability, aligning with the region’s emphasis on Good Manufacturing Practice (GMP) compliance and product traceability.
The increasing adoption of cryopreservation enabled allogeneic cell therapies and expansion of commercial cell banks drive market growth. The presence of established cryogenic infrastructure, along with collaborations between academic medical centers and biotech firms, supports the large scale storage of clinical grade cells and tissues. Initiatives led by the National Institutes of Health (NIH) to standardize cell storage protocols and quality testing have further strengthened the U.S. position as a global leader in cell cryopreservation innovation and biobanking capacity.
Asia Pacific Cell Cryopreservation Insights
Asia Pacific region is anticipated to register the fastest CAGR of 13.48% during the forecast period. The driving factor for this growth is the rapid development of regional stem cell banking networks and government backed regenerative medicine programs across major countries. Nations such as China, Japan, and South Korea are establishing large scale cryogenic facilities integrated with advanced data management systems to support national cell therapy initiatives and clinical research programs.
The increasing government emphasis on affordable regenerative healthcare and academic industry collaborations drives the market growth. Supported by initiatives like the “National Biopharma Mission” and BIRAC funding, Indian biotechnology firms are developing indigenous cryoprotectants and low cost storage systems tailored to tropical conditions. Furthermore, public-private partnerships are enabling the establishment of regional cryo-biobanks for hematopoietic and mesenchymal stem cells, positioning India as a cost-competitive hub for cryopreserved cell resources.
By Region Market Share (in percent share %), 2025
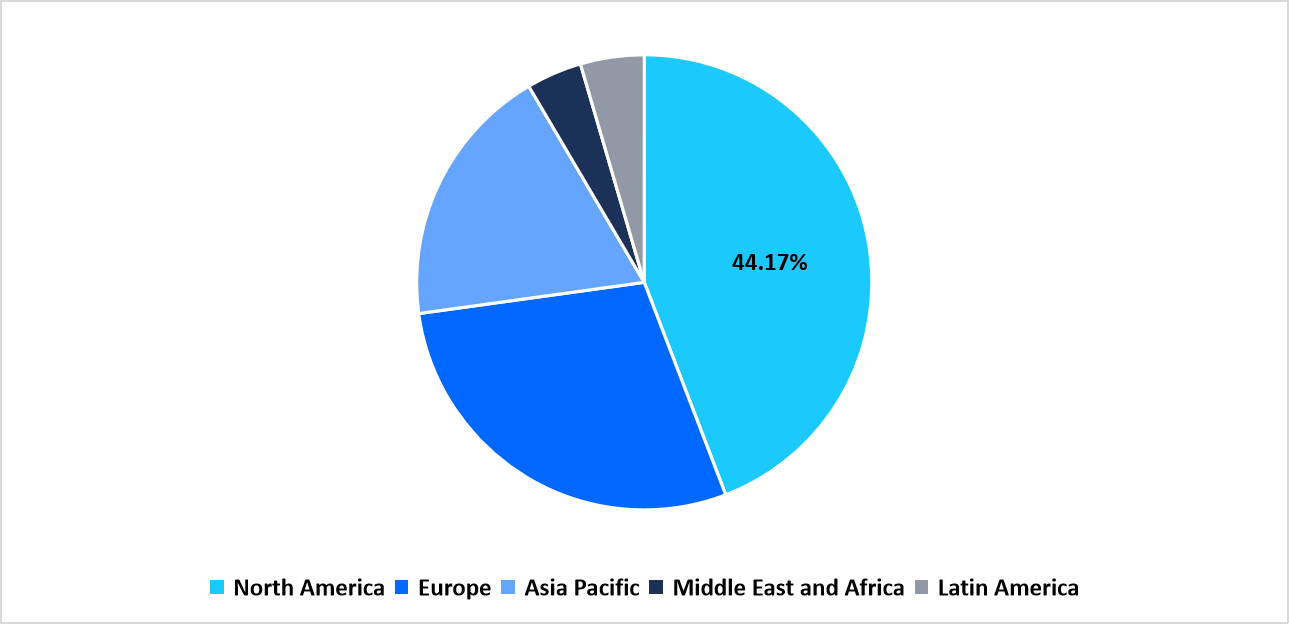
Source: Straits Research
Europe Cell Cryopreservation Insights
Europe’s market growth is driven by stringent regulatory frameworks emphasizing standardized cryogenic preservation for advanced therapy medicinal products (ATMPs). The European Medicines Agency (EMA) and the European Society for Cell and Gene Therapy (ESGCT) have established technical guidelines promoting validated cryopreservation processes for clinical-grade cell batches. This harmonized regulatory landscape ensures reproducibility and cell integrity, attracting investment from both established pharmaceutical manufacturers and emerging biotech firms developing next-generation cell-based therapies.
The increasing expansion of academic-industrial partnerships to commercialize cryopreservation media formulations drives market growth. German universities and research consortia, supported by federal grants, are collaborating with local biotech startups to optimize serum-free and animal-component-free cryomedia for stem cell and CAR T cell applications. This strong integration between R&D and manufacturing infrastructure strengthens Germany’s position as a European leader in advanced cryopreservation solutions.
Latin America Cell Cryopreservation Insights
The Latin American cell cryopreservation market is expanding due to growing government investment in regenerative medicine and national biobank programs. Countries such as Brazil, Mexico, and Argentina are building local cryogenic storage facilities to support research in genetic disorders, immunotherapies, and fertility preservation. The adoption of region-specific protocols for low-temperature logistics and preservation of umbilical cord stem cells is enhancing biobanking accessibility and clinical readiness.
The establishment of public stem cell repositories and academic biobanks drives market growth. Supported by the Brazilian Ministry of Health and national research agencies, universities are implementing GMP-compliant cryopreservation labs integrated with public hospitals. This initiative improves patient access to cryopreserved stem cells for future regenerative therapies and reinforces Brazil’s role as a regional hub for cell banking and translational research.
Middle East and Africa Cell Cryopreservation Insights
The Middle East and Africa region is witnessing major growth due to increasing cross-border collaborations and infrastructure development in cellular biotechnology. Regional governments are investing in biobanking networks and cryogenic logistics to support cancer immunotherapy and reproductive medicine. Strategic alliances with global cryopreservation technology providers are enhancing capacity for temperature-controlled storage and transport of biological materials across the region.
The expansion of national biorepositories and fertility preservation centers drives market growth. The UAE government’s “National Genome Strategy” and partnerships with international biopharma companies have facilitated the creation of state-of-the-art cryogenic storage facilities within Dubai and Abu Dhabi. These initiatives support long term preservation of stem cells and genetic materials, fostering medical tourism and regional leadership in precision and regenerative medicine.
Type Insights
The cryoprotectant agents segment dominated the market in 2025, owing to their essential role in maintaining cell viability during freezing and thawing processes. Dimethyl Sulfoxide (DMSO) remained the most widely used agent due to its superior permeability and ability to minimize intracellular ice formation. Glycerol and other emerging cryoprotectants are increasingly being explored for specialized applications where reduced cytotoxicity and improved biocompatibility are required.
The equipment segment is anticipated to register the fastest CAGR of 12.32% during the forecast period, driven by technological advancements in automated freezing systems, programmable cryogenic freezers, and smart liquid nitrogen supply tanks. The growing demand for precision-controlled cryogenic storage infrastructure in biobanks and pharmaceutical research facilities further accelerates the adoption of advanced cryopreservation equipment.
Application Insights
The stem cells segment dominated the market in 2025, with a revenue share of 33.24%, attributed to the extensive use of cryopreservation in regenerative medicine, stem cell banking, and cell-based research. Growing clinical trials involving hematopoietic and mesenchymal stem cells for therapeutic use further contributed to this dominance.
The oocytes and embryonic cells segment is projected to record the fastest CAGR of 12.24% during the forecast period, supported by the increasing adoption of fertility preservation techniques and advancements in assisted reproductive technologies (ART). The rising awareness of fertility preservation among cancer patients and women delaying childbirth further boosts demand in this segment.
By Application Market Share (in percent share %), 2025

Source: Straits Research
End-User Insights
The biopharmaceutical and pharmaceutical companies segment dominated the global cell cryopreservation market in 2025, accounting for a revenue share of 41.23%. This growth is attributed to the increasing use of cryopreservation for maintaining cell line integrity, ensuring consistent biomanufacturing processes, and supporting cell-based therapeutic development.
The biobanks segment is anticipated to register the fastest CAGR of 12.12% during the forecast period, driven by the expanding number of public and private biorepositories, growing demand for long-term storage of biological samples, and the rise in personalized medicine and precision diagnostics initiatives worldwide.
Competitive Landscape
The global cell cryopreservation market is moderately fragmented, consisting of a mix of established life science companies and emerging biotechnology firms focusing on advanced cryopreservation media, cell storage solutions, and biobanking services.
Forever Labs: An emerging market player
Forever Labs, a U.S.-based biotechnology company founded in 2015, specializes in adult stem cell harvesting and long-term cryopreservation services. The company’s proprietary process enabled the extraction, processing, and storage of bone marrow-derived mesenchymal stem cells in clinical-grade cryogenic biorepositories for future therapeutic use. By focusing on personalized cell preservation and regenerative medicine, Forever Labs positions itself as a promising innovator in the cell cryopreservation domain.
List of Key and Emerging Players in Cell Cryopreservation Market
- Lonza
- Thermo Fisher Scientific Inc.
- Merck KGaA
- Sartorius AG
- BioLife Solutions, Inc.
- Corning Incorporated
- PromoCell
- HiMedia Laboratories
- DH Life Sciences, LLC.
- Eppendorf SE
- GE HealthCare
- Azenta US Inc.
- Cryoport, Inc.
- STEMCELL Technologies
- Creative Biolabs
- Miltenyi Biotec GmbH
- Avantor, Inc.
- FUJIFILM Irvine Scientific d/b/a FUJIFILM Biosciences
- ZENOGEN PHARMA CO., LTD.
- WAK-Chemie Medical GmbH
- Others
Strategic Initiatives
- October 2025: Cryoport, Inc. announced the opening of a new 55,000 square foot Global Supply Chain Center (GSCC) in Louvres (near Paris, France) to support advanced therapies, including cryopreservation services of cell/gene therapies and biologics.
- March 2025: Alpha Teknova, Inc. and Pluristyx, Inc. announced the launch of their proprietary PluriFreeze cryopreservation system, which is an entirely synthetic, animal origin free solution (consisting of a protective wash “PluriFreeze Base” and a freezing medium “PluriFreeze PF10”) designed for allogeneic cell therapy workflows.
- September 2024: Pharmacosmos A/S introduced “PentaHibe Complete”, a ready-to-use cGMP-compliant cryopreservation medium, which represented an innovation in simplifying cryopreservation workflows for cell based applications.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 3.30 Billion |
| Market Size in 2026 | USD 3.67 Billion |
| Market Size in 2034 | USD 8.68 Billion |
| CAGR | 11.37% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product Type, By Application, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Cell Cryopreservation Market Segments
By Product Type
-
Cryoprotectant Agents
- Glycerol
- Dimethyl Sulfoxide (DMSO)
- Others
-
Equipment
- Incubators
- Liquid Nitrogen Supply Tanks
- Freezers
- Others
By Application
- Stem Cells
- Oocytes and Embryotic cells
- Sperm cells
- Hepatocytes
- Others
By End User
- Biopharmaceutical & Pharmaceutical Companies
- Research Institutes
- Biobanks
- IVF clinics
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































