Cell & Gene Therapy Clinical Trials Market Size, Share & Trends Analysis Report By Phase (Phase I, Phase II, Phase III, Phase IV), By Indication (Oncology, Cardiology, Musculoskeletal, Infectious Diseases, Dermatology, Immunology & Inflammation, Opthalmology, Hematology, Gastroenterology, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Cell & Gene Therapy Clinical Trials Market Overview
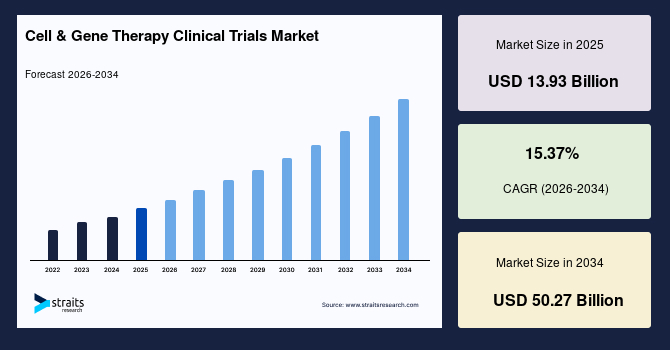
The global cell & gene therapy clinical trials market size is estimated at USD 13.93 billion in 2025 and is projected to reach USD 50.27 billion by 2034, growing at a CAGR of 15.37% during the forecast period. Sustained growth of the market is propelled by the increasing number of clinical trials that play an essential role in the growth of the market due to their role in validating the safety, efficacy, and therapeutic potential of new treatments. The table below depicts the data of clinical trials of cell therapy from 2024 to H1 2025.
Table: Clinical trials for cell therapy
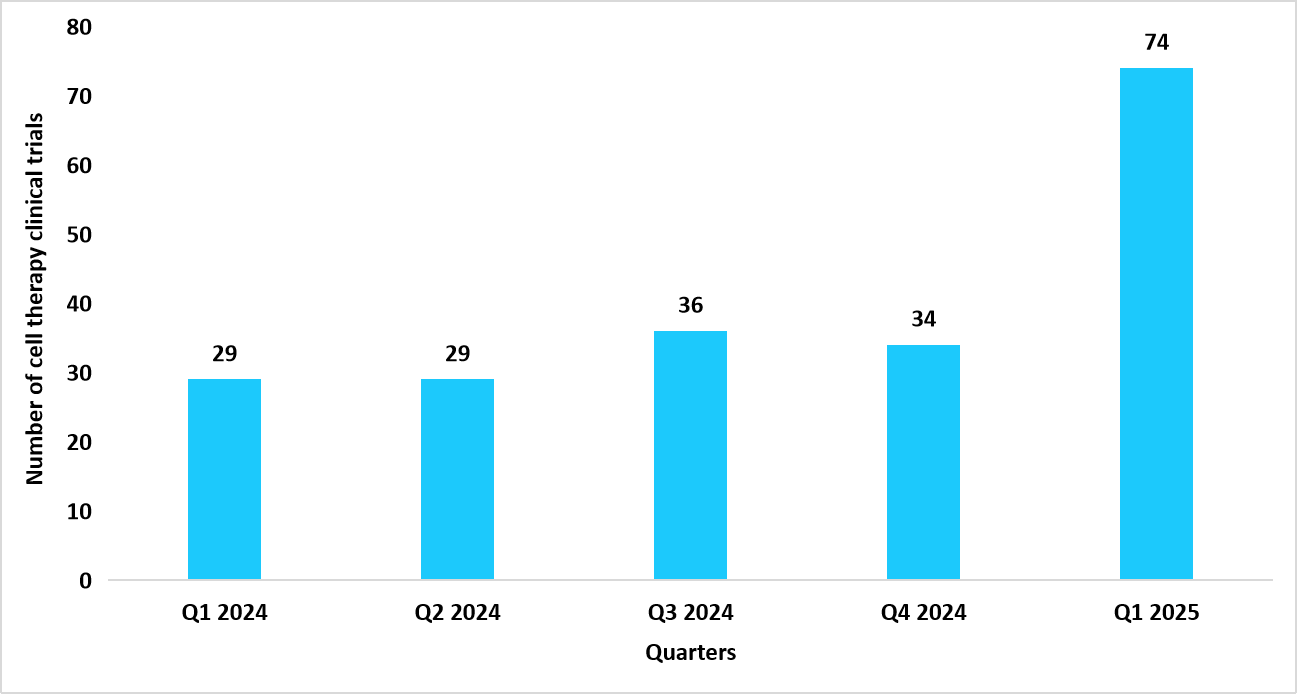
Source: Alliance for Regenerative Medicine
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 43.17%.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 17.24%.
- Based on Phase, the phase III segment dominated the market in 2025 with a revenue share of 59.28%.
- Based on the Indication, the hematology segment is expected to register the fastest CAGR growth of 16.12%.
- The U.S. dominates the global cell & gene therapy clinical trials market, valued at USD 4.70 billion in 2024 and reaching USD 5.41 billion in 2025.
Market Size & Forecast
- 2025 Market Size: USD 13.93 billion
- 2034 Projected Market Size: USD 50.27 billion
- CAGR (2025 to 2034): 15.37%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The cell & gene therapy clinical trials market encompasses the research and development activities aimed at evaluating the safety, efficacy, and long-term outcomes of innovative therapeutic interventions that modify cellular and genetic materials to treat, prevent, or potentially cure various diseases. This market is segmented based on clinical trial phases, such as Phase I, Phase II, Phase III, and Phase IV, with each representing a distinct stage of product testing, from initial human safety assessment to post-marketing surveillance.
Additionally, it is classified by indication, covering a wide spectrum of therapeutic areas including oncology, cardiology, musculoskeletal disorders, infectious diseases, dermatology, immunology and inflammation, ophthalmology, hematology, gastroenterology, and others. The market reflects the expanding pipeline of advanced therapies, increasing regulatory support, and growing clinical success rates across multiple disease domains, positioning cell and gene therapy as a transformative segment within the broader biotechnology and pharmaceutical landscape.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
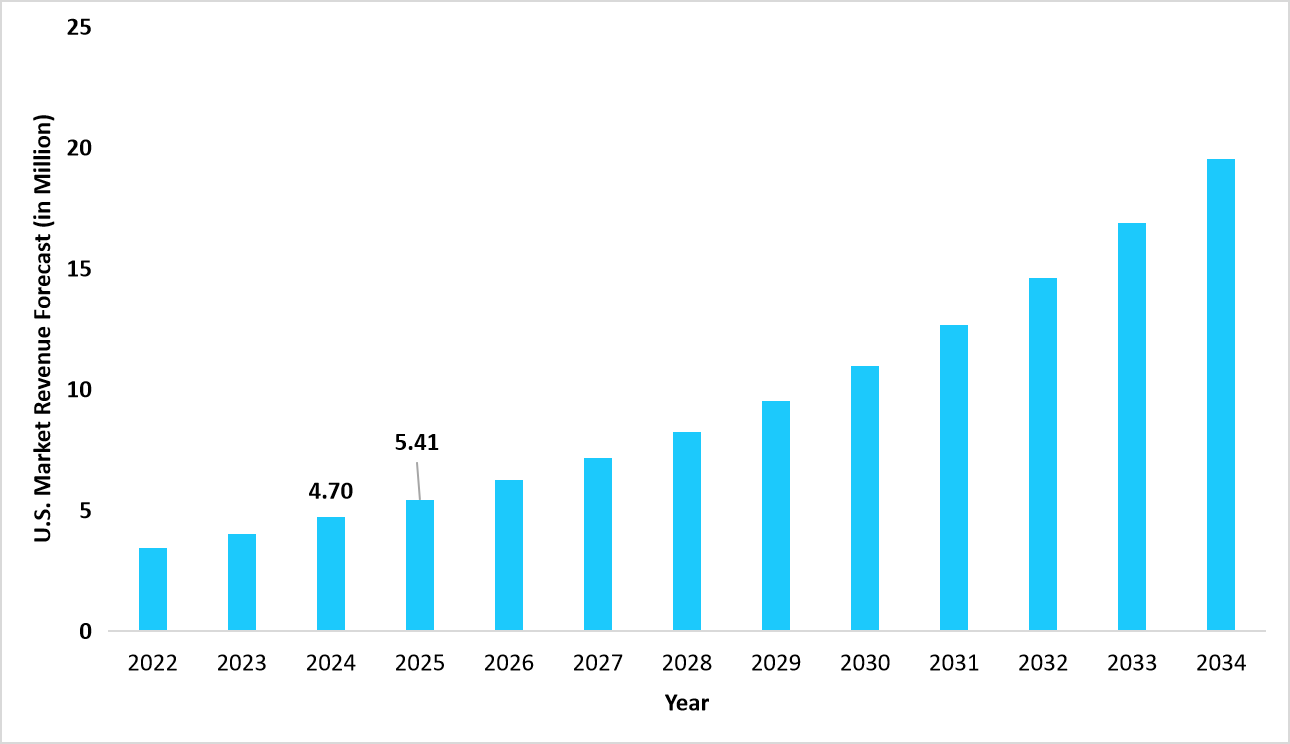
Source: Straits Research
Market Trends
Shift from Traditional Large-Scale Trials Towards Advanced, Adaptive Trial Designs for Rare Diseases
The cell and gene therapy (CGT) clinical trial landscape is witnessing a shift from conventional, large-scale randomized controlled trials to more adaptive, patient-centric designs tailored for small and rare disease populations. Recently, the U.S. Food and Drug Administration (FDA) released a draft guidance titled “Innovative Designs for Clinical Trials of Cellular and Gene Therapy Products in Small Populations”, encouraging the use of flexible methodologies such as single-arm studies, Bayesian models, and externally controlled trials. This transition aimed to accelerate therapy development while maintaining data integrity and patient safety. Such a transformative shift supported faster approvals and more inclusive participation in rare disease trials, marking a crucial evolution in the CGT clinical research framework.
Transition Towards Allogeneic “Off-the-Shelf” and Commercially Viable Platforms from Autologous Cell Therapies
The movement from personalized autologous therapies, which require cells derived from each individual patient, to allogeneic “off-the-shelf” therapies that use donor-derived cells for standardized production and broader clinical accessibility, enhances the market growth. Recently, Longeveron Inc. announced positive Phase 2a results for laromestrocel, an allogeneic cell therapy for mild Alzheimer’s disease, and reported a Type B meeting with the FDA alongside RMAT and Fast Track designations. This reflected the industry’s strategic shift toward therapies that can be produced, stored, and delivered more efficiently, enhancing their commercial viability and patient reach.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 13.93 Billion |
| Estimated 2026 Value | USD 16.02 Billion |
| Projected 2034 Value | USD 50.27 Billion |
| CAGR (2026-2034) | 15.37% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Novartis AG, Gilead Sciences, Inc. , CRISPR Therapeutics, Intellia Therapeutics, Inc., Editas Medicine |
to learn more about this report Download Free Sample Report
Market Driver
Technological Advancements Accelerating the Development of Advanced Cell and Gene Therapies
A key driver of the cell and gene therapy (CGT) clinical trials market is the rapid advancement in genomic editing technologies and delivery platforms that enhance the precision, safety, and scalability of therapeutic development. For example, in March 2025, CRISPR Therapeutics announced the initiation of an advanced CRISPR Cas12a-based clinical trial targeting beta-thalassemia, offering improved gene editing efficiency with reduced off-target effects. Such innovations improved trial success rates and expanded the therapeutic pipeline for complex genetic, oncological, and rare diseases. These technological advancements not only accelerate the transition of therapies from preclinical to clinical stages but also strengthen confidence among investors and regulatory bodies, thereby driving overall market growth.
Market Restraints
Ethical and Compliance Challenges Impede Progress of Cell and Gene Therapy Clinical Trials
A major restraint in the cell and gene therapy clinical trials market is the complex ethical considerations and compliance requirements associated with trial approvals and patient safety. According to the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), CGT trials demand extensive preclinical validation, long-term safety monitoring, and strict manufacturing standards due to the high-risk nature of genetic modification and cell-based treatments. These factors often led to prolonged approval timelines, increased operational costs, and challenges in scaling clinical programs, particularly for smaller biotechnology firms. Such ethical and compliance hurdles hindered the pace of innovation and delayed the translation of advanced therapies from research to clinical application, thereby restraining overall market growth.
Market Opportunity
Growing Government and Institutional Funding Creating Strong Opportunities for Cell and Gene Therapy Clinical Trials
A major opportunity for the cell and gene therapy (CGT) clinical trials market lies in the major funding and investments provided by governments and international organizations to accelerate research and therapeutic advancements in regenerative medicine. For instance, in January 2025, the U.S. National Institutes of Health (NIH) announced a funding allocation of USD 950 million under the Somatic Cell Genome Editing (SCGE) program to support large-scale clinical trials and translational research focused on rare genetic disorders and oncology. This funding strengthened the clinical trial ecosystem by enhancing infrastructure, supporting biotech collaborations, and expanding patient recruitment networks. Such initiatives created substantial growth opportunities for research institutions and biopharmaceutical companies to advance novel CGT-based therapies and accelerate their path toward commercialization.
Regional Analysis
The North America region dominated the market with a revenue share of 43.17% in 2025. The growth is attributed to the strong presence of advanced clinical infrastructure and specialized trial networks for rare and orphan diseases. The region has a growing network of dedicated gene and cell therapy research firms, such as the National Institutes of Health (NIH) Clinical Center and Alliance for Regenerative Medicine (ARM) that enabled seamless collaboration between biotech startups, academic institutions, and regulatory bodies.
A key driver for the U.S. cell and gene therapy clinical trials market is the strong flow of venture capital and government funding dedicated to advancing regenerative medicine. Programs such as the ARPA-H (Advanced Research Projects Agency for Health) and state innovation grants actively support research in gene editing, cell engineering, and viral vector technologies. This potent funding environment enables biotech startups and research institutions to accelerate early-stage discoveries into clinical trials, fostering innovation and strengthening the U.S. position as a global hub for cell and gene therapy development.
Asia Pacific Market Insights
The Asia Pacific region is projected to grow at the fastest CAGR of 17.24% during the forecast period, driven by the rapid expansion of regional biomanufacturing capabilities and local partnerships for clinical trial execution. Countries such as China, Japan, South Korea, and Singapore are investing heavily in advanced bioprocessing facilities, viral vector production, and contract research infrastructure, which attract global biotech firms to conduct early- and mid-stage trials locally. This strong manufacturing and regulatory ecosystem is accelerating the region’s role in global cell and gene therapy development.
India Market: The market in India is driven by the rising prevalence of genetic and rare diseases coupled with improved patient awareness and diagnostic capabilities. The increasing availability of advanced genomic testing and precision diagnostics in major hospitals and private labs is enabling early identification of eligible patients for cell and gene therapy trials. This growing patient pool, along with greater acceptance of personalized medicine, is creating a strong foundation for the expansion of clinical research and development activities in India’s cell and gene therapy clinical trials sector.
By Region Market Share (in percent share %), 2025
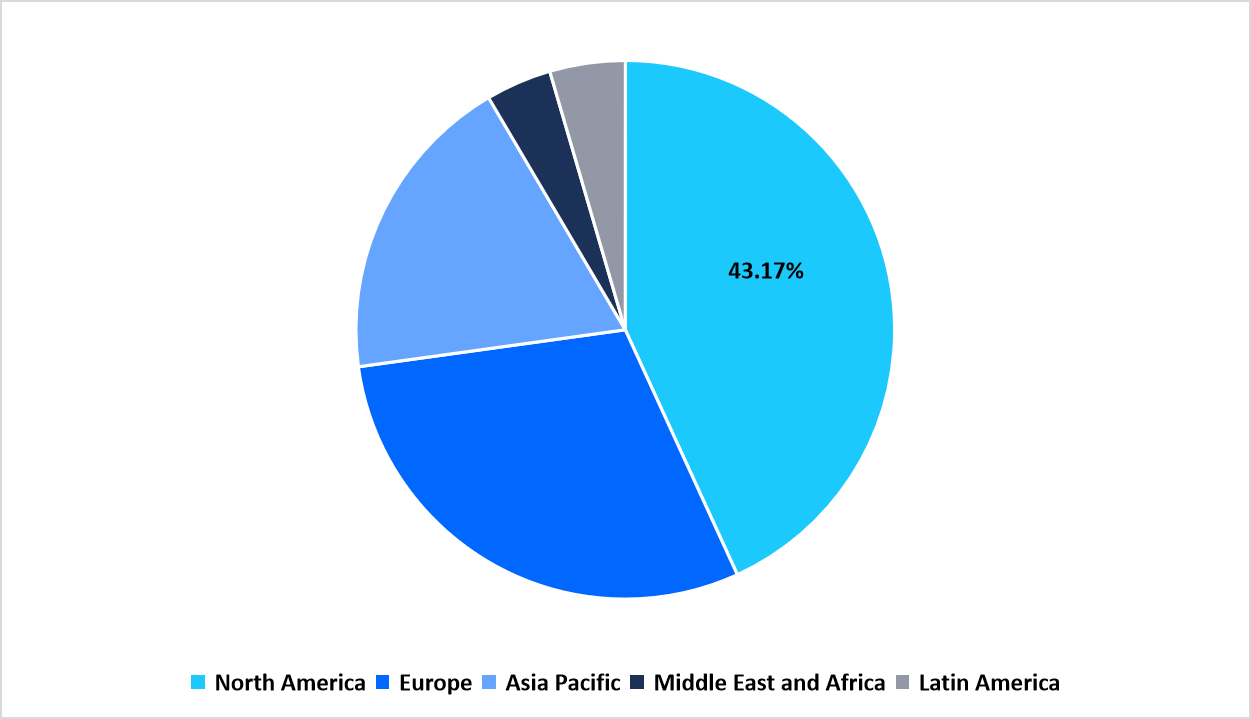
Source: Straits Research
Europe Market Insights
The European market is driven by the increasing investment in advanced therapy manufacturing hubs and specialized clinical trial facilities. Countries such as Germany, the UK, and the Netherlands are developing dedicated GMP-certified cell and gene therapy centers that support end-to-end clinical development, from vector production to patient delivery.
The UK market is growing rapidly, driven by the expansion of dedicated cell and gene therapy innovation hubs. For example, the Cell and Gene Therapy Catapult in London supports clinical manufacturing and trial development for advanced therapies, supporting companies like Orchard Therapeutics and Autolus Therapeutics to accelerate their clinical programs within the UK.
Middle East and Africa Market Insights:
The Middle East and Africa market is expanding steadily, driven by the rising government focus on developing precision medicine and genomic research infrastructure. Countries such as Saudi Arabia and the United Arab Emirates are launching national genome programs and investing in biobanking and advanced medical research centers to support personalized treatment approaches. These initiatives are laying the network for introducing and conducting cell and gene therapy clinical trials across the region, particularly for inherited and rare genetic disorders.
The South African market is growing due to the government’s efforts to strengthen regulatory frameworks and streamline approval processes for advanced therapies. The South African Health Products Regulatory Authority (SAHPRA) introduced updated guidelines to facilitate the evaluation of gene and cell-based treatments, making it easier for global and local sponsors to initiate clinical trials. This regulatory modernization created a more supportive environment for innovation and attracting investment in the country’s emerging biotechnology sector.
Latin America Market Insights
The Latin American market is expanding due to the growing participation of regional hospitals in multinational clinical trials. Countries like Brazil and Mexico are increasingly serving as trial sites for global biotech firms, leveraging their large patient pools and improving research infrastructure to support cell and gene therapy studies.
The Argentine market is growing due to rising government incentives for biotechnology innovation, including tax benefits and research grants that encourage local startups and universities to engage in cell and gene therapy development and clinical research.
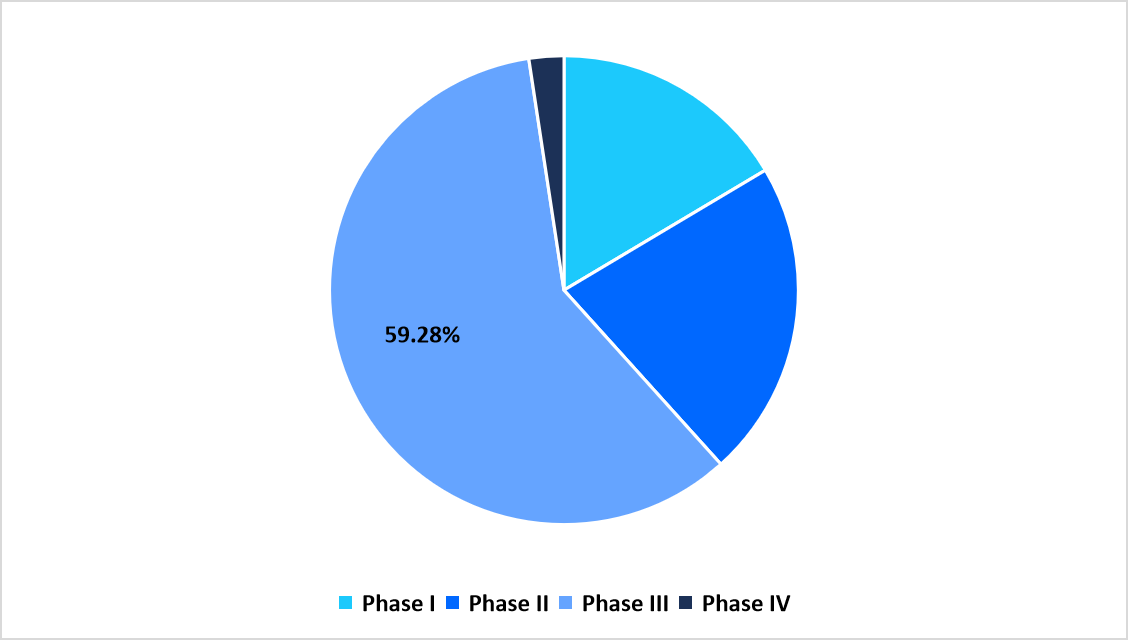
Phase Insights
The phase III segment dominated the market with 59.28% share, due to the increasing number of late-stage pipeline candidates transitioning from early-phase success. As cell and gene therapies advance through regulatory pathways, Phase III trials serve as the critical stage for evaluating efficacy, long-term safety, and comparative efficiency at scale.
The phase II segment is anticipated to register the fastest CAGR of 16.27%, driven by the rising transition of promising early-stage therapies into mid-stage trials, supported by favourable regulatory designations such as FDA Breakthrough Therapy and EMA PRIME status.
By Phase Market Share (in percent share %), 2025
Source: Straits Research
Indication Insights
The oncology segment dominated the market in 2025, with a revenue share of 48.37%, due to the high number of cell and gene therapy clinical trials targeting various cancers, including leukemia, lymphoma, and solid tumors. The growing success of CAR-T and TCR-based therapies, along with increasing regulatory approvals and strong investment in cancer immunotherapy research, further strengthened oncology’s leading position in the market.
The hematology segment is anticipated to register the fastest CAGR of 16.12% during the forecast period, owing to the rising number of clinical trials targeting inherited blood disorders such as sickle cell disease, beta thalassemia, and haemophilia.
Competitive Landscape
The global cell and gene therapy clinical trials market is moderately fragmented, with a mix of established biopharmaceutical leaders and emerging biotechnology startups driving innovation across various therapeutic areas, including oncology, rare genetic disorders, and regenerative medicine.
- Vironexis Biotherapeutics: An emerging market player
- Vironexis Biotherapeutics, a U.S.-based gene therapy startup, received FDA approval to start its first clinical trial for VNX-101, a new cancer treatment that used a harmless virus (AAV) to deliver a gene supporting the immune system's fight against acute lymphoblastic leukemia (ALL).
List of Key and Emerging Players in Cell & Gene Therapy Clinical Trials Market
- Novartis AG
- Gilead Sciences, Inc.
- CRISPR Therapeutics
- Intellia Therapeutics, Inc.
- Editas Medicine
- bluebird bio, Inc.
- Sarepta Therapeutics, Inc.
- ALLOGENE THERAPEUTICS
- Adaptimmune
- Beam Therapeutics
- Orchard Therapeutics plc
- REGENXBIO Inc.
- uniQure NV.
- Rocket Pharmaceuticals
- Abeona Therapeutics Inc.
- Krystal Biotech, Inc.
- MeiraGTx Limited.
- Bristol-Myers Squibb Company
- Pfizer Inc.
- Takeda Pharmaceutical Company Limited
- Others
Strategic Initiatives
- March 2025: Bharat Biotech announced the launch of its first dedicated cell and gene therapy facility in Telangana, with an investment of USD 75 million, with an aim of launching two cell therapies and three gene therapies by around 2028, which target oncology and rare disease indications.
- January 2025: Genenta Science expanded its partnership with AGC Biologics by setting up a dedicated cell and gene therapy production area at AGC’s facility in Milan, Italy. This new space supported Genenta in manufacturing its cell therapy products for ongoing clinical trials in brain cancer (glioblastoma) and kidney cancer. The company planned to produce around 27 batches of its experimental therapies in 2025.
- April 2024: Labcor announced the expansion of its precision oncology portfolio to advance drug development programs via supporting biopharma, pharmaceutical and clinical research.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 13.93 Billion |
| Market Size in 2026 | USD 16.02 Billion |
| Market Size in 2034 | USD 50.27 Billion |
| CAGR | 15.37% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Phase, By Indication |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Cell & Gene Therapy Clinical Trials Market Segments
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Indication
- Oncology
- Cardiology
- Musculoskeletal
- Infectious Diseases
- Dermatology
- Immunology & Inflammation
- Opthalmology
- Hematology
- Gastroenterology
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































