HIV Clinical Trials Market Size, Share & Trends Analysis Report By Phase (Phase I, Phase II, Phase III, Phase IV), By Study Design (Interventional Studies, Observational Studies, Expanded Access Studies), By Sponsor (Pharmaceutical & Biopharmaceutical Companies, Non-Profit Organizations, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
HIV Clinical Trials Market Overview
The global HIV clinical trials market size is estimated at USD 1.64 billion in 2025 and is projected to reach USD 2.71 billion by 2034, growing at a CAGR of 5.78% during the forecast period. The Substantial market growth is attributed to the adoption of AI-driven patient matching systems, which enhanced recruitment efficiency and optimized outcomes in HIV clinical trials.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 45.84% share in 2025.
- The Asia Pacific region is estimated to grow at the fastest pace, with a CAGR of 7.06% during the forecast period.
- Based on phase, the phase II segment dominated the market with 31.67% revenue share in 2025.
- By study design, interventional studies dominated the market with a revenue share of 65.72% in 2025.
- By sponsor, the pharmaceutical & biopharmaceutical companies segment dominated the market in 2025.
- The U.S. dominates the market, valued at USD 662.62 million in 2024 and reaching USD 698.07 million in 2025.
Table: U.S. HIV Clinical Trials Market Size (USD Million)
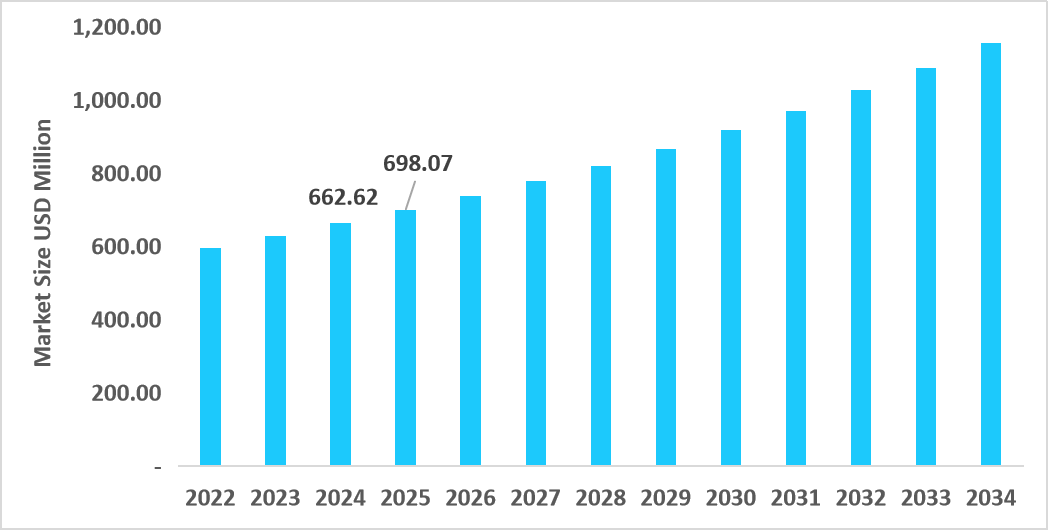
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.64 billion
- 2034 Projected Market Size: USD 2.71 billion
- CAGR (2026-2034): 5.78%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The HIV clinical trials market comprises various phases, such as Phase I, Phase II, Phase III, and Phase IV trials, each focusing on evaluating safety, efficacy, and outcomes of investigational therapies. By study design, the market comprises interventional, observational, and expanded access studies, reflecting diverse research methodologies. Furthermore, the market involves various sponsors such as pharmaceutical and biopharmaceutical companies, non-profit organizations, and other institutions.
Latest Market Trends
Growth of Pediatric-Focused HIV Clinical Trials
A key trend in the HIV clinical trials market is the increasing emphasis on pediatric populations. For example, ViiV Healthcare initiated the ODYSSEY trial to evaluate optimized antiretroviral regimens in children living with HIV. Focusing on pediatric studies addresses unmet treatment needs, ensures age-appropriate formulations, and expands the market by supporting safer, more effective therapies for younger patients globally.
Rise of Decentralized and Virtual Clinical Trials
Major HIV clinical trial sponsors, including Merck & Co. Inc. and Janssen Pharmaceuticals, are adopting decentralized and virtual trial models to improve patient recruitment and retention. By using telemedicine, remote monitoring, and digital data collection, these trials reduce geographic barriers and enhance participant engagement. This trend enables faster study execution and broader population access, positioning companies to strengthen their competitive presence in the global market.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.64 Billion |
| Estimated 2026 Value | USD 1.73 Billion |
| Projected 2034 Value | USD 2.71 Billion |
| CAGR (2026-2034) | 5.78% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Gilead Sciences, ViiV Healthcare, GSK plc, Johnson & Johnson, Merck & Co. Inc. |
to learn more about this report Download Free Sample Report
HIV Clinical Trials Market Drivers
Growing Investment in the Development of Long-Acting Antiretroviral Therapies
The surge in development of antiretroviral therapies is a key driver for market growth. The increasing prevalence of HIV and the need for improved patient adherence accelerated the development of long-acting injectable therapies. In 2024, ViiV Healthcare advanced its cabotegravir-based regimen through multiple global Phase III trials, while Gilead Sciences expanded its long-acting portfolio with novel investigational agents. These developments demonstrate that companies are prioritizing these therapies to reduce dosing frequency and address unmet clinical needs, thereby driving the growth of the HIV clinical trials market.
Market Restraints
Limited patient recruitment and high dropout rates
Recruiting participants for long-term and multiphase studies is challenging due to geographic barriers and strict eligibility criteria. High dropout rates and difficulties in enrolling diverse populations slow trial progress, increase costs, and limit the ability of sponsors to generate robust data. Recently, a CHOICES study aimed to evaluate extended-release naltrexone (XR-NTX) for opioid use disorder in HIV positive individuals, faced barriers, including stigma associated with HIV and substance use.
Thus, limited patient recruitment and high dropout rates pose a barrier to market growth and the timely development of new HIV therapies.
Market Opportunity
Development of Functional Vaccines
Developing therapeutic vaccines aimed at a functional HIV cure presents a major opportunity for market growth. Current antiretroviral therapies effectively suppress HIV but cannot eradicate the virus, requiring lifelong treatment. Companies focusing on vaccines that target latent viral reservoirs address this critical unmet need.
Therefore, investing in functional cure strategies allows manufacturers to develop new candidates and conduct clinical trials of these vaccine candidates.
Regional Analysis
North America accounted for the 45.84% market share in the global market in 2025. This dominance is accelerated by strong government and institutional funding support that sustains large-scale research initiatives. Agencies such as the National Institute of Health (NIH) and the National Institute of Allergy and Infectious Diseases (NIAID) consistently invest in vaccine and cure-focused programs. This robust funding ecosystem accelerates innovation and reinforces North America’s position as a global leader in HIV research and therapeutic development.
The growth of the U.S. HIV clinical trials market is accelerated by robust public-private research collaborations between the NIH, academic institutions, and leading pharmaceutical companies. These partnerships accelerate translational research, enable extensive patient recruitment, and foster innovation in therapies such as gene editing and therapeutic vaccines.
Asia Pacific Market Insights
Asia Pacific is emerging as the fastest-growing region in the HIV clinical trials market with a CAGR of 7.06% from 2026 to 2034, owing to the rising participation in decentralized and community-based trials in urban and semi-urban areas of India and Thailand. This approach enhances patient accessibility, improves retention rates, and allows for real data collection, for faster evaluation of innovative HIV therapies and supporting the region’s emergence as a key hub for clinical research.
Australia's market growth is supported by its strong integration of Aboriginal and Torres Strait Islander health programs into research initiatives. This inclusion ensures culturally sensitive trial designs, improves participation from underserved populations, and generates valuable epidemiological insights, which assist in addressing regional health disparities and advancing inclusive clinical research practices.
Regional Market share (%) in 2025
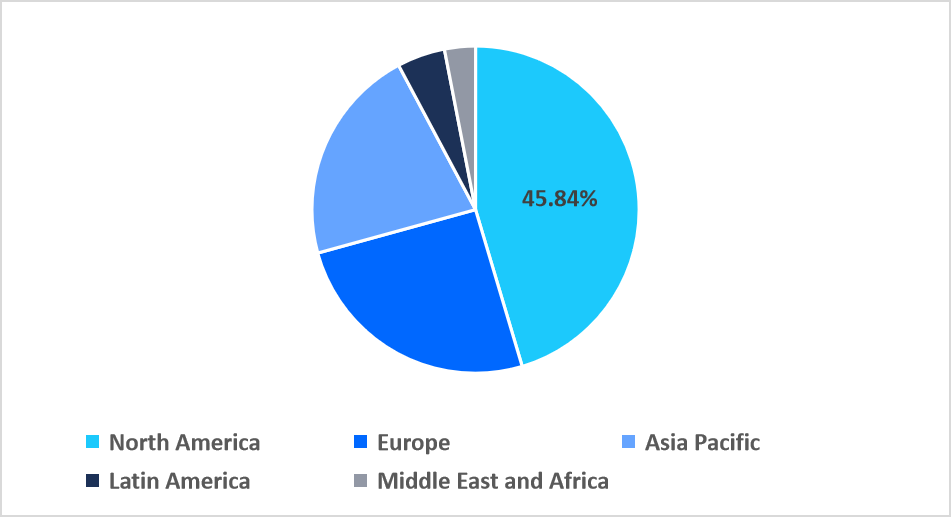
Source: Straits Research
Europe Market Insights
Europe's HIV clinical trials market growth is driven by the expansion of pan-European research consortia and interregional clinical networks, such as the EAVI2020 and EuroCoord initiatives. These initiatives streamline multi-country trial execution, enhance data harmonization, and strengthen regulatory alignment across EU member states, accelerating the development of novel therapies, improving patient diversity, and scientific exchange across the European HIV research ecosystem.
The UK market is witnessing growth due to the establishment of advanced genomic research hubs, such as those supported by Genomics England and the UK Biobank. These research hubs enable precise patient stratification and biomarker identification, fostering innovation in personalized HIV treatment approaches and supporting the rapid development of targeted therapeutic candidates across the country.
Latin America Market Insights
The Latin America HIV clinical trials market growth is augmented by the establishment of a regional pharmacogenomic research project in Brazil and Argentina, which focuses on genetic diversity among HIV patients. These programs elevate understanding of drug response variations, support population-specific therapy development, and increase lead development for clinical research.
The growth of Argentina's market is strengthened by its centralized electronic health record (EHR) networks, which allow researchers to efficiently track patient histories, monitor treatment adherence, and identify eligible participants for clinical studies. This advanced digital system accelerates trial recruitment, which, in turn, supports the market growth.
Middle East and Africa Market Insights
The Middle East and Africa HIV clinical trials market is expanding due to regional biobanking and sample-sharing networks, particularly in South Africa and Kenya. These initiatives facilitate access to diverse genetic and viral samples, support collaborative research across borders, and accelerate the development of diagnostic and therapeutic tools tailored to local populations.
The global market growth in Saudi Arabia is supported by the country’s investment in specialized infectious disease research centers, such as the King Fahd Medical Research Center. These centers provide advanced laboratory infrastructure and foster collaboration with international HIV research networks.
Phase Insights
The phase II segment dominated the market with a revenue share of 31.67% in 2025. This growth is driven by a focus on adaptive trial designs, which allow researchers to modify dosing, cohort size, or treatment arms based on interim results. This flexibility accelerates efficacy evaluation, reduces resource waste, and enables precise identification of promising therapies before advancing to larger, costlier Phase III trials.
The phase I segment is projected to register the fastest CAGR growth of 6.03% during the forecast period, owing to the increasing use of humanized in vitro models and organ-on-chip technologies. These innovations allow early assessment of safety and pharmacokinetics in a more physiologically relevant setting and accelerate candidate selection.
By Phase Market Share (%), 2025
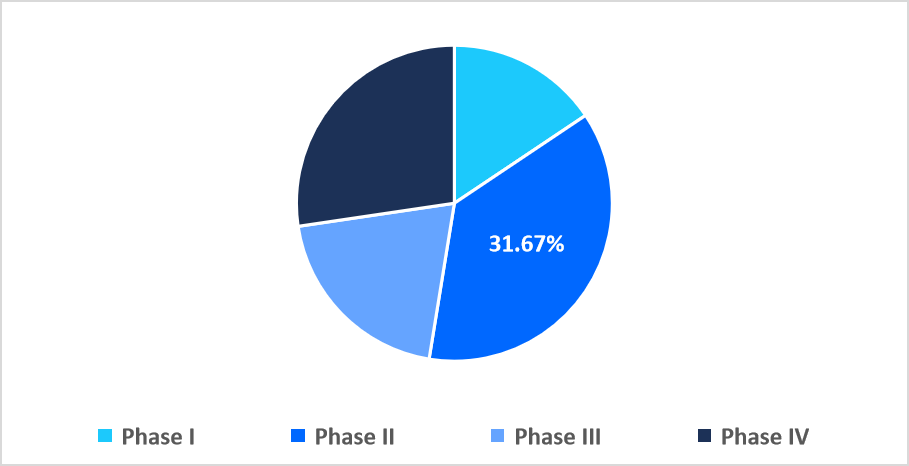
Source: Straits Research
Study Design Insights
The interventional studies segment dominated the market in 2025 with a revenue share of 65.72%. This dominance is attributed to the rising adoption of adaptive platform trial designs. These studies allow multiple HIV therapies to be evaluated simultaneously within a single protocol, validating rapid comparison, efficient resource utilization, and dynamic modification of treatment arms based on interim results, thereby accelerating the identification of effective interventions.
The expanded access studies segment is estimated to grow at the fastest CAGR of 6.49% during the forecast period. This growth is supported by increasing compassionate use programs for investigational HIV therapies. These programs allow patients with limited treatment options to access promising drugs early and strengthen regulatory support for broader clinical development and market approval.
Sponsor Insights
The pharmaceutical & biopharmaceutical companies segment dominated the market in 2025. This growth is augmented by strategic investment in AI-supported drug repurposing for HIV therapies. By leveraging computational models to identify existing compounds with potential anti-HIV activity, these companies accelerate lead development, reduce costs, and enhance the probability of clinical success.
The non-profit organizations segment is expected to register a CAGR of 6.18% during the forecast period, owing to their growing role in decentralized community-led HIV trials. By leveraging local clinics and outreach networks, non-profit organizations improve trial accessibility for hard-to-reach populations, enhance participant diversity, and generate efficacy data, thereby supporting HIV research.
Competitive Landscape
The global HIV clinical trials market is moderately consolidated, with a mix of major pharmaceutical companies, biotechs, and research institutions driving innovation. Key players include Gilead Sciences, ViiV Healthcare, Merck & Co., and others, which dominate through extensive R&D pipelines and global trial networks. These companies remain competitive by focusing on strategic collaborations, accelerating vaccine research, and expanding access programs to strengthen their global presence and therapeutic portfolios.
HOOKIPA Pharma: An emerging market player
HOOKIPA Pharma Inc. is an innovative biotechnology company actively engaged in advancing HIV therapeutic vaccine development. The company is gaining recognition through its collaboration with Gilead Sciences for its novel vaccine candidate, HB-500.
- In July 2024, HOOKIPA initiated a Phase 1b clinical trial to evaluate the safety, tolerability, and immunogenicity of HB-500, marking a notable step toward achieving a functional cure for HIV.
List of Key and Emerging Players in HIV Clinical Trials Market
- Gilead Sciences
- ViiV Healthcare
- GSK plc
- Johnson & Johnson
- Merck & Co. Inc.
- Pfizer Inc.
- Bristol Myers Squibb
- AbbVie Inc.
- Sanofi
- PPD Inc.
- IQVIA Inc.
- ICON plc
- WuXi AppTec
- Bionor Holding AS
- Charles River Laboratories
- Syneos Health
- BioNTech SE
- Sangamo Therapeutics
- Parexel International Corporation
- Others
Strategic Initiatives
- September 2025: UNAIDS announced two new agreements to advance progress in stopping new HIV infections. Under this agreement, UNITAID, the Clinton Health Access Initiative (CHAI), and Wits RHI are providing financial, technical, and regulatory support to Indian generic manufacturer Dr Reddy’s Laboratories.
- September 2025: Gilead Sciences entered into a partnership with PEPFAR to deliver twice-yearly Lenacapavir for HIV Prevention for up to Two Million People in low and middle-income countries.
- August 2025: Wistar Institute received the National Institutes of Health (NIH) grant, $17 million research award to launch the iCure Consortium to develop individualized “cure regimens” for HIV.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.64 Billion |
| Market Size in 2026 | USD 1.73 Billion |
| Market Size in 2034 | USD 2.71 Billion |
| CAGR | 5.78% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Phase, By Study Design, By Sponsor |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
HIV Clinical Trials Market Segments
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional Studies
- Observational Studies
- Expanded Access Studies
By Sponsor
- Pharmaceutical & Biopharmaceutical Companies
- Non-Profit Organizations
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































