Life Sciences Translation Services Market Size, Share & Trends Analysis Report By Type (Clinical/Document Translation, Technical Translation, Labeling and Device Translation, Corporate/Marketing Translation), By Category (Manual, Technology/AI-Based), By Application (Clinical Trials, Regulatory Submissions, Pharmaceutical Documentation, Patient-Reported Outcomes, Marketing Authorization), By End Use (Pharmaceutical Manufacturers, Biotechnology Companies, Medical Device Manufacturers, Clinical Research Organizations, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Life Sciences Translation Services Market Overview
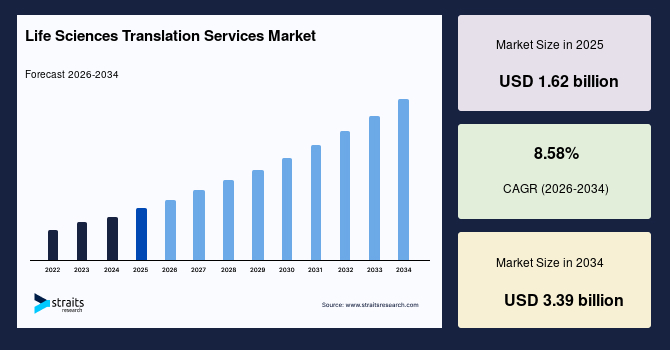
The global life sciences translation services market size is valued at USD 1.62 billion in 2025 and is estimated to reach USD 3.39 billion by 2034, growing at a CAGR of 8.58% during the forecast period. The market growth is accelerated by the rising volumes of multinational clinical trials and regulatory submissions that require precise multilingual documentation across diverse regulatory jurisdictions.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 41.23% share in 2025.
- The Asia Pacific region is forecasted to grow at the fastest pace, with a CAGR of 10.58%during the forecast timeframe.
- Based on Type, technical translation segment dominated the market with a revenue share of 38.73% in 2025.
- Based on Category, manual segment is anticipated to register the fastest CAGR of 9.45% during the forecast period.
- Based on Application, clinical trials segment dominated the market with a revenue share of 32.32% in 2025.
- Based on End Use, clinical research organizations segment dominated the market with a revenue share of 25.62% in 2025.
- The U.S. dominates the market, valued at USD 558.15 million in 2024 and reaching USD 603.64 million in 2025.
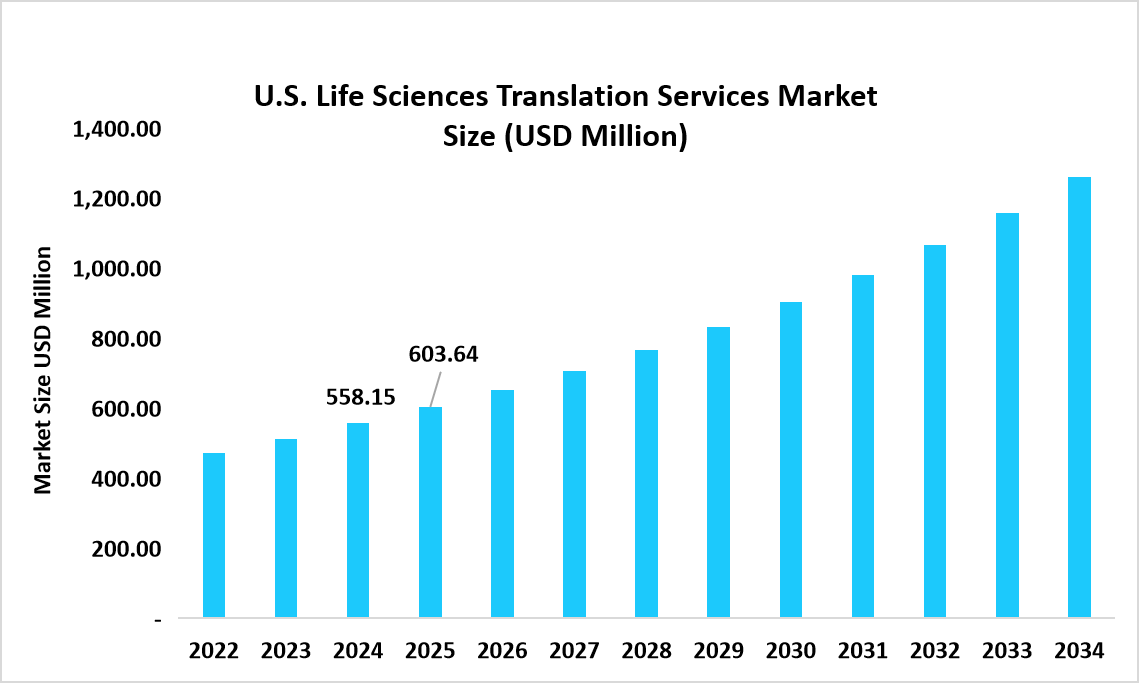
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.62 billion
- 2034 Projected Market Size: USD 3.39 billion
- CAGR (2026-2034): 8.58%
- Dominating Region: North America
- Fastest-Growing Region: Asia Pacific
The life sciences translation services market comprises specialized language services designed to support regulated healthcare and biomedical activities by converting scientific medical and regulatory content across languages while preserving technical accuracy and compliance. The market addresses translation requirements for clinical and document content technical and engineering files product labeling and medical device documentation as well as corporate and marketing materials. Service delivery spans manual linguist-led translation and technology or AI based solutions integrated within regulated workflows. Applications include multilingual support for clinical trials regulatory submissions pharmaceutical documentation patient reported outcomes and marketing authorization dossiers. End users include pharmaceutical manufacturers biotechnology companies medical device manufacturers clinical research organizations and other healthcare stakeholders that operate across international markets and require compliant consistent and standardized communication throughout the product development and lifecycle management process.
Market Trends
Shift From Standalone Document Translation To Embedded Language Workflows Within Regulated Platforms
The market is witnessing a shift from isolated document level translation toward translation functions embedded directly within clinical safety regulatory and quality management systems. Life sciences organizations increasingly align language processing with structured workflows for adverse event intake protocol amendments and regulatory lifecycle management. This shift supports standardized terminology control version tracking and audit readiness across multilingual documentation environments without treating translation as a separate operational step.
Shift From General Language Service Models To Domain-Specific Life Sciences Linguistics
There is a shift from broad based translation vendors toward providers with deep expertise in clinical research regulatory affairs and pharmacovigilance terminology. Sponsors and CROs prioritize linguists trained in therapeutic area specific content controlled vocabularies and regulatory writing conventions. This transition reflects growing sensitivity to linguistic accuracy in safety narratives labeling text and patient facing materials governed by strict compliance expectations.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.62 billion |
| Estimated 2026 Value | USD 1.75 billion |
| Projected 2034 Value | USD 3.39 billion |
| CAGR (2026-2034) | 8.58% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Vistatec, Language Scientific, TransPerfect Life Sciences, RWS Group, Summa Linguae Technology |
to learn more about this report Download Free Sample Report
Market Driver
Rising Regulatory Scrutiny On Multilingual Safety And Clinical Documentation
Increasing regulatory oversight on global clinical trials and post marketing safety reporting is driving demand for compliant life sciences translation services. Regulatory agencies require precise localized documentation aligned with standardized medical terminology and reporting formats. In 2024 safety communication and guidance updates published by the U.S. Food and Drug Administration, emphasis was placed on consistent adverse event reporting quality across global trials which reinforced sponsor investment in structured multilingual translation processes integrated into pharmacovigilance operations.
Market Restraint
High Validation And Compliance Burden For Language Technologies
Adoption of advanced translation tools within life sciences environments faces restraint from validation requirements under regulated quality systems. Translation workflows integrated into clinical and safety platforms require extensive documentation testing and audit trails to meet regulatory expectations. This compliance burden increases implementation timelines and operational costs particularly for smaller service providers and emerging sponsors.
Market Opportunity
Expansion of Decentralized Trials And Remote Patient Engagement Materials
Growth in decentralized and hybrid clinical trial models presents an opportunity for specialized translation services focused on patient facing digital content. Expansion of remote consent electronic diaries and multilingual patient communications increases demand for linguistically accurate culturally aligned translations. Providers that align translation services with digital trial platforms and patient engagement technologies are positioned to capture emerging demand driven by evolving clinical research models.
Regional Analysis
North America led the life sciences translation services market in 2025 with a share of 41.23% in 2025, supported by high volumes of multinational clinical trials and centralized regulatory and pharmacovigilance operations. Regulatory submissions to the U.S. Food and Drug Administration required multilingual clinical documentation safety narratives and labeling content to support global development and commercialization strategies, sustaining regional demand.
In the U.S., adoption increased through integration of translation workflows into clinical research and safety operations. According to a 2024 report published by IQVIA, the company expanded multilingual content management and localization support across its clinical trial and safety services portfolio to support global study execution and regulatory submissions, reinforcing demand for specialized life sciences translation services within FDA regulated environments.
Asia Pacific Life Sciences Translation Services Market Insights
Asia Pacific emerged as a fast growing region due to rising clinical trial activity across China India and South Korea. Regional regulators required localized language submissions for trial approvals patient materials and safety updates, driving demand for regional linguistic expertise aligned with life sciences standards.
China demonstrated growth supported by regulatory reforms that increased clinical trial filings and post marketing safety reporting, prompting pharmaceutical companies to invest in Chinese language translation for regulatory dossiers informed consent forms and adverse event documentation.
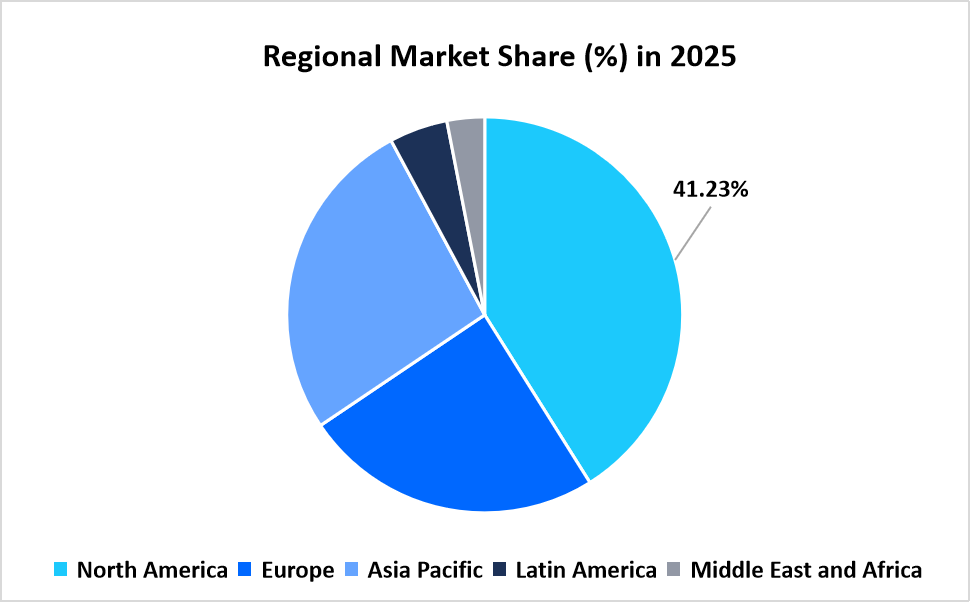
Source: Straits Research
Europe Market Insights
Europe experienced steady growth driven by multilingual regulatory requirements under the European Medicines Agency framework. Marketing authorization applications variations and pharmacovigilance submissions required parallel translations across multiple EU member states, increasing structured demand for compliant language services.
France recorded expansion due to strong biopharmaceutical manufacturing activity and growing volumes of clinical research coordinated by academic hospitals and contract research organizations. French sponsors increasingly outsourced translation of clinical protocols investigator brochures and safety documentation to align with EMA and national authority requirements.
Latin America Market Insights
Latin America recorded consistent expansion supported by increased participation in multinational clinical trials and regulatory submissions across Spanish and Portuguese speaking markets. Regulatory agencies required translated clinical protocols safety narratives and patient facing documentation to support trial approvals and pharmacovigilance activities.
Mexico showed growth as regulatory filings to COFEPRIS increased alongside expansion of clinical research centers, driving outsourcing of compliant medical and regulatory translation services.
Middle East and Africa Market Insights
The Middle East and Africa market expanded as governments increased investments in clinical research infrastructure and strengthened regulatory oversight. Multilingual documentation requirements across Arabic English and French supported demand for compliant translation services.
The United Arab Emirates experienced growth due to expansion of clinical research hubs and regulatory alignment initiatives requiring Arabic translations of clinical trial documentation safety reports and product information, increasing utilization of life sciences translation services across the region.
Type Insights
The technical translation segment dominated the market in 2025 with a revenue share of 38.73%. Dominance aligned with rising volumes of medical device documentation, manufacturing specifications validation reports and quality system records requiring precise terminology and compliance aligned language conversion across jurisdictions.
The clinical document translation segment is projected to record the fastest growth at 9.12%, supported by increasing clinical trial activity and rising demand for localized protocols investigator brochures informed consent forms and safety narratives across multinational study sites.
Category Insights
Technology and AI based translation dominated the market, reflecting adoption of automated language processing within pharmacovigilance regulatory and clinical documentation workflows. Enterprises integrated AI driven translation to manage scale consistency and turnaround across global operations.
Manual translation is expected to grow at the fastest rate of 9.45%, driven by continued reliance on human linguistic expertise for high risk regulatory submissions patient facing content and complex scientific narratives requiring contextual accuracy.
Application Insights
Clinical trials represented the leading application segment with a market share of 32.32% in 2025. Growth aligned with increased global trial decentralization, requiring multilingual documentation for site activation patient engagement and safety reporting.
Marketing authorization translation is anticipated to expand at the fastest pace with a CAGR of 9.78%, supported by rising numbers of simultaneous product filings across regions that require synchronized multilingual dossiers labeling summaries and risk management plans.
End Use Insights
Clinical research organizations dominated the market with a revenue share of 25.62%, driven by their role in managing cross border trials regulatory coordination and centralized documentation workflows for sponsors.
Pharmaceutical manufacturers are expected to register the fastest growth at 9.88%, supported by expansion of in house regulatory operations lifecycle management activities and post approval documentation requiring continuous multilingual translation support.
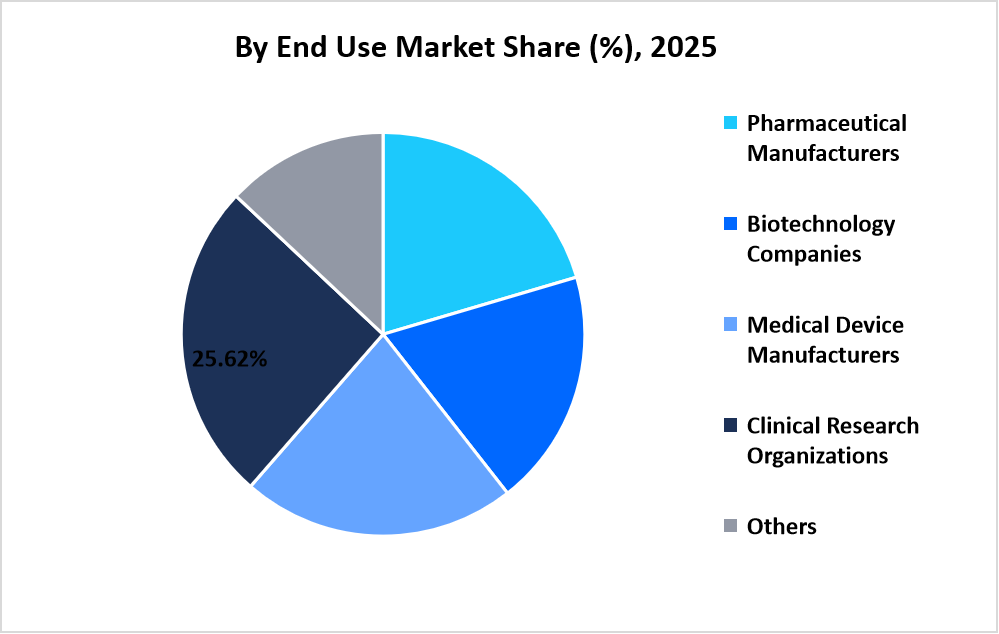
Source: Straits Research
Competitive Landscape
The life sciences translation services market reflected moderate consolidation, with large technology enabled service providers and enterprise software companies strengthening language automation capabilities within regulated healthcare workflows. Established players focused on integrating AI driven translation into pharmacovigilance regulatory and clinical documentation environments to support global operations across multiple jurisdictions.
-
Oracle: An emerging market player
Oracle maintained a competitive position in the life sciences translation services landscape through embedded AI powered translation functionality within its pharmacovigilance platforms. The company focused on automating adverse event report translation to streamline safety case intake and processing while reducing manual workload.
- In October 2024, Oracle introduced new AI powered features in Oracle Argus Safety to automate the translation of adverse event reports. The enhancements accelerated safety case intake and processing, reduced manual effort, and improved consistency across global pharmacovigilance operations.
List of Key and Emerging Players in Life Sciences Translation Services Market
- Vistatec
- Language Scientific
- TransPerfect Life Sciences
- RWS Group
- Summa Linguae Technology
- Skrivanek
- Europe Localize
- marstranslation
- BayanTech
- Lionbridge Technologies, LLC.
- Conversis
- Morningside, Inc
- Crimson Interactive Inc.
- Welocalize Life Sciences
- ALM Translations Ltd
- Stepes
- Questel
- BURG Translations
- Others
Recent Developments
- December 2025: Sesen announced the launch of TrialS, a next-generation regulatory translation validation platform designed to support life sciences organizations achieve greater accuracy, consistency, and regulatory alignment across global clinical and labeling submissions.
- June 2025: SESEN launched as a specialized language services provider focused on the life sciences sector. The company offered translation and localization services for clinical, regulatory, and commercial content.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.62 billion |
| Market Size in 2026 | USD 1.75 billion |
| Market Size in 2034 | USD 3.39 billion |
| CAGR | 8.58% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Category, By Application, By End Use |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Life Sciences Translation Services Market Segments
By Type
- Clinical/Document Translation
- Technical Translation
- Labeling and Device Translation
- Corporate/Marketing Translation
By Category
- Manual
- Technology/AI-Based
By Application
- Clinical Trials
- Regulatory Submissions
- Pharmaceutical Documentation
- Patient-Reported Outcomes
- Marketing Authorization
By End Use
- Pharmaceutical Manufacturers
- Biotechnology Companies
- Medical Device Manufacturers
- Clinical Research Organizations
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































