Liver Cirrhosis Treatment Market Size, Share & Trends Analysis Report By Treatment Type (Immunosuppressants, Antibiotics, Vaccines, Corticosteroids, Other Treatment Types), By Disease Type (Alcoholic Cirrhosis, Atrophic Cirrhosis, Biliary Cirrhosis, Cryptogenic Cirrhosis), By Distribution Channel (Hospital Pharmacies, Drug Stores & Retail Pharmacies, Other Distribution Channel) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Liver Cirrhosis Treatment Market Size
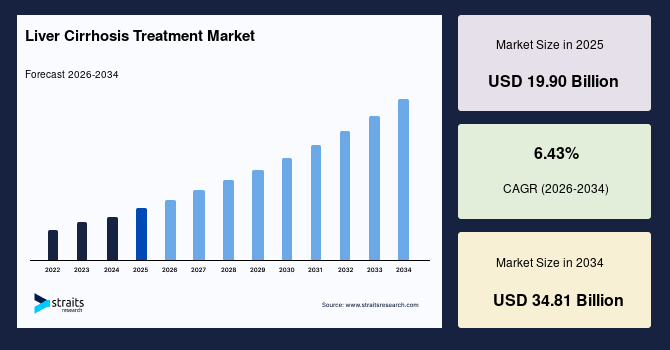
The liver cirrhosis treatment market size was valued at USD 19.90 billion in 2025 and is estimated to reach USD 34.81 billion by 2034, growing at a CAGR of 6.43% during the forecast period (2026-2034). The demand for immunosuppressants and corticosteroids is growing to manage the increased liver congestion that leads to liver cirrhosis. These drugs reduce liver inflammation and suppress the immune system’s attack on liver tissue.
Key Market Insights
- North America dominated the liver cirrhosis treatment market with the largest market share of 42.33% in 2025.
- Asia Pacific is expected to be the fastest-growing region in the liver cirrhosis treatment market during the forecast period at a CAGR of 7.80%.
- By treatment type, the immunosuppressants segment dominated the market with the largest share of 36.20% in 2025.
- By disease type, the alcoholic cirrhosis segment accounted for the largest market share of 46.52% in 2025.
- By distribution channel, the drug stores & retail pharmacies segment is expected to grow rapidly during the forecast period at a CAGR of 7.11%.
- The US liver cirrhosis treatment market size was valued at USD 7.67 billion in 2025 and is projected to reach USD 8.14 billion in 2026.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 19.90 Billion |
| Estimated 2026 Value | USD 21.15 Billion |
| Projected 2034 Value | USD 34.81 Billion |
| CAGR (2026-2034) | 6.43% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Lilly, Amgen Inc., AstraZeneca, Bayer AG, Bristol-Myers Squibb Company |
to learn more about this report Download Free Sample Report
Liver Cirrhosis Treatment Market Trends
Expansion of disease-modifying and combination drug therapies
The liver cirrhosis treatment market is undergoing a transition from managing symptoms to disease-modifying therapies, which focus on the underlying cause of liver injury. These medications reverse the fibrosis of the liver that contributes to the progression of cirrhosis. For instance, resmetirom, a thyroid hormone receptor-β agonist, received approval in 2024 for the treatment of NASH and demonstrated efficacy in decreasing liver fat and fibrosis, thereby preventing the progression of the disease to advanced cirrhosis. These medications are increasingly used in combination with traditional therapies such as lactulose and rifaximin to manage symptoms.
Advancements in therapeutic drug development targeting fibrosis and cirrhosis
The development of therapeutic drugs for liver cirrhosis is now targeting the fibrosis and biological processes that cause liver injury. Unlike conventional treatments that only target alleviating symptoms, these disease-modifying medications are now designed to slow down the scarring of the liver. For example, a new class of drugs, efruxifermin, developed by Akero Therapeutics, showed promising Phase 2b clinical trial results. In this clinical trial, patients with cirrhosis due to metabolic liver disease showed significant improvement in liver fibrosis compared to the placebo group. Therefore, the development of disease-modifying therapies is a key trend driving market growth.
Liver Cirrhosis Treatment Market Drivers
Rising prevalence of metabolic and viral liver diseases boosts market growth
The growing incidence of metabolic and viral liver diseases across the globe is a key factor propelling the market for liver cirrhosis treatment. NAFLD and NASH are escalating at a rapid pace due to rising levels of obesity and diabetes. For example, NASH is one of the leading reasons for liver transplants in the US. On the other hand, hepatitis B and C infections are highly prevalent on the Asian and African continents. This steady increase in cirrhosis cases is increasing the patient base to develop new treatments and fueling the demand for treatment options across the globe.
Shortage of liver transplants drives demand for medical therapies
The gap between the demand for liver transplants and the supply of available organs is leading to growing demand for medicines. In the US, more than 10,000 patients are added to the waiting list for a liver transplant every year, yet only 9,000 liver transplants are carried out annually. Therefore, most patients suffering from cirrhosis are undergoing drug treatment to control the progression of the disease. This leads to advancements in the pharmacological interventions, thereby creating a high demand for cirrhosis and antifibrotic drugs.
Market Restraints
High treatment costs are limiting access to effective therapies to high treatment cost limits access to effective therapies
The high cost of advanced therapies is a major factor limiting access to advanced therapies in low-income countries. Many novel antifibrotic and regenerative treatments are expensive to develop and prescribe, restricting access in low- and middle-income countries. For example, the cost of medications for advanced liver cirrhosis cases exceeds USD 3,000 monthly in the US. These high costs of treatments decrease their adoption in low-disposable-income countries, thereby restraining market growth.
Market Opportunities
Expansion of early-stage therapeutic intervention through integrated care models offers growth opportunities
A key opportunity in the liver cirrhosis treatment market is the expansion of therapeutic intervention programs that combine disease screening with drug treatment. For instance, the US Veterans Health Administration used digital care coordination to spread awareness on hepatitis C antiviral treatment nationwide, achieving cure rates above 95% and identifying cirrhosis patients earlier. This model increased demand for cirrhosis-specific medications to manage fibrosis progression and complications, creating sustained opportunities for market growth.
Technological Landscape
- Lunit INSIGHT Liver is a deep learning algorithm used for liver disease screening in CT and MRI scans.
- iCAD ProFound AI liver modules integrate deep learning algorithms for cross-modality liver imaging research.
- Aidoc AI Abdominal Imaging uses CT scan analysis for diagnosing liver abnormalities.
Regional Analysis
The liver cirrhosis treatment market in North America held a leading position in 2025 with a market share of 42.33%, supported by high disposable income, maximum diagnostic penetration, and structured clinical pathways for chronic liver disease management.
The US accounted for the largest market share due to the FDA’s accelerated approval of semaglutide for the treatment of metabolic dysfunction-associated steatohepatitis (MASH) with moderate to advanced liver fibrosis, which has opened a new therapeutic standard in the US, extending the treatment options beyond the conventional antifibrotics. The FDA approval aims at a huge patient pool of approximately 22 million Americans suffering from MASH.
Asia Pacific
Asia Pacific is emerging as a fast-expanding region for liver cirrhosis management with a CAGR of 7.80%, driven by a high prevalence of chronic hepatitis infections and a rising incidence of metabolic liver disorders. For instance, about 75 million people are living with hepatitis B and C in Southeast Asia, which drives the need for diagnosis and treatment services, thereby fuels demand for liver cirrhosis therapeutics.
China leads the Asia Pacific region with the largest market share in 2025, due to the government’s focus on expanding the scope of basic medical insurance for major antiviral drugs, including hepatitis B medications such as entecavir (ETV) and tenofovir (TDF). This increases access for millions of patients at risk of cirrhosis. These antiviral drugs became widely covered by insurance, which increased their use from 14% to nearly 80% among treated HBV patients and decreased the development of fibrosis and cirrhosis. This initiative addresses the demand for therapies related to the management of liver diseases.
Europe
Europe continued to record steady adoption of liver cirrhosis diagnostics and treatment solutions, supported by universal healthcare systems and coordinated liver disease surveillance programs. Regional policies promoting early detection of hepatitis B, hepatitis C, and alcohol-related liver disease support consistent demand for diagnostic testing and long-term disease management.
Germany led the European market due to high availability of specialist hepatology services, strong hospital infrastructure, and widespread use of non-invasive liver assessment technologies in routine clinical practice. For example, tertiary care centers across Germany have widely adopted transient elastography and advanced imaging-based fibrosis assessment to diagnose and monitor liver cirrhosis, enabling earlier treatment initiation and optimized therapeutic management.
Latin America
Latin America demonstrated steady growth in the liver cirrhosis market, as liver disease continues to be the leading cause of cirrhosis in Brazil, Mexico, Argentina, and Chile, accounting for over half of the total number of cirrhosis cases. This has led to a continued high demand for hospitalization and specialized treatment, thus ensuring sustained market growth for drugs that address complications arising from fibrosis.
Brazil dominated the regional landscape through public sector investment in specialty liver care centers and improved access to antiviral therapies, supporting long-term management of cirrhosis-related complications. For example, government-backed programs have expanded the availability of hepatitis antiviral treatment within public hospitals, helping reduce disease progression and improve outcomes for patients with cirrhosis across the country.
Middle East and Africa
The Middle East and Africa market progressed through government-supported healthcare modernization initiatives and increased focus on non-communicable diseases. National liver disease awareness campaigns and expansion of tertiary care hospitals improved access to diagnostic services and clinical monitoring.
South Africa held a leading position in the region due to the ongoing digitization of hospital systems and strengthening of hepatology services across public healthcare facilities. For example, major public hospitals have implemented electronic health record systems and digital liver disease monitoring tools to improve early diagnosis, treatment coordination, and long-term management of liver cirrhosis patients.
Treatment Type Insights
The immunosuppressants segment dominated the liver cirrhosis treatment market and accounted for a 36.20% revenue share in 2025. This is because the targeted use of immunosuppressive treatment has increased in cases of autoimmune cirrhosis. Immunosuppressants have shown transplant-free survival and slowing of disease progression, thus dominating the treatment type segment.
The corticosteroids segment is projected to grow at a CAGR of 6.94% during the forecast period, owing to the increasing clinical use of budesonide for autoimmune hepatitis patients without advanced cirrhosis, where it achieves higher biochemical remission rates and fewer systemic side effects than traditional steroids, expanding its adoption in targeted patient groups.
Disease Type Insights
The alcoholic cirrhosis segment accounted for the 46.52% market share in 2025, due to the rising number of cases of NAFLD, NASH, and chronic hepatitis infections, which are contributing to the rising number of liver cirrhosis cases. The rising number of patients is fueling the demand for treatment options, thereby offering substantial growth opportunities to the liver cirrhosis treatment market.
The cryptogenic cirrhosis segment is expected to register a CAGR of 7.05% during the forecast period. This growth is propelled by increased use of histopathology and molecular profiling after liver transplantation, which identifies previously unrecognized disease origins, expanding confirmed cryptogenic diagnoses and related treatment demand.
Distribution Channel Insights
The hospital pharmacies segment dominated the market with a revenue share of 51.78% in 2025, due to their direct integration with inpatient therapeutic drug monitoring systems for dose adjustments of hepatotoxic and narrow therapeutic index drugs in cirrhosis patients. Moreover, easy drug availability and free consultation with doctors also make hospital pharmacies a primary distribution channel.
The drug stores & retail pharmacies segment is projected to grow at the fastest CAGR during the forecast timeframe due to increasing outpatient management of stable cirrhosis patients, where maintenance therapies such as antibiotics, vaccines, and supportive medications are routinely refilled outside hospital settings, improving convenience, adherence, and continuity of care.
| SEGMENT | INCLUSION | DOMINANT SEGMENT | SHARE OF DOMINANT SEGMENT, 2025 |
|---|---|---|---|
|
TREATMENT TYPE |
|
Immunosuppressants |
36.20% |
|
DISEASE TYPE |
|
Alcoholic Cirrhosis |
46.52% |
|
DISTRIBUTION CHANNEL |
|
Hospital Pharmacies |
51.78% |
|
REGION |
|
North America |
42.33% |
Regulatory Bodies Governing Liver Cirrhosis Treatment Market
| REGULATORY BODY | COUNTRY/REGION |
|---|---|
|
US Food and Drug Administration |
US |
|
European Medicines Agency |
Europe |
|
Pharmaceutical and Medical Devices Agency |
Japan |
|
Therapeutic Goods Administration |
Australia |
|
Central Drugs Standard Control Organization |
India |
Competitive Landscape
The liver cirrhosis treatment market is moderately fragmented, with competition from large multinational pharmaceutical companies, biotech companies, specialty hepatology drug developers, and emerging clinical innovators. Large companies compete with research and development pipelines and established hospital networks, while smaller biotech companies are engaged in developing innovative antifibrotic and disease-modifying therapies. The intensity of competition is fueled by factors such as the success of clinical trials, regulatory approvals, therapeutic effectiveness, safety, and pricing. Emerging trends in the competitive environment include rising investments in fibrosis-targeting drugs, the development of combination therapies, and partnerships.
List of Key and Emerging Players in Liver Cirrhosis Treatment Market
- Lilly
- Amgen Inc.
- AstraZeneca
- Bayer AG
- Bristol-Myers Squibb Company
- Novo Nordisk
- Hoffmann-La Roche Ltd.
- Madrigal Pharmaceuticals
- Gilead Sciences, Inc.
- GSK plc
- Johnson & Johnson Services Inc.
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi
- Takeda Pharmaceutical Company Limited
- Sumitomo Pharma Co., Ltd.
- Intercept Pharmaceuticals Inc.
- Alnylam Pharmaceuticals, Inc.
- Biogen Inc.
- Bausch Health Companies Inc.
- Cytotheryx
- Sagimet Biosciences
Latest News on Key and Emerging Players
| TIMELINE | COMPANY | DEVELOPMENT |
|---|---|---|
|
January 2026 |
Cytotheryx |
Cytotheryx received USD 60 million in financing to advance its research and development. |
|
December 2025 |
Sagimet Biosciences |
The company announced positive Phase 1 PK data for the Denifanstat and Resmetirom combination, supporting plans for regulatory consultation. |
|
October 2025 |
Novo Nordisk |
The company agreed to acquire Akero Therapeutics, which offers efruxifermin (FGF21 analogue in Phase 3 for MASH and compensated cirrhosis). This broadens Novo’s access to metabolic disease, including liver scarring/fibrosis. |
|
August 2025 |
Madrigal Pharmaceuticals |
The company received European Commission conditional approval for Rezdiffra to treat metabolic dysfunction-associated steatohepatitis (MASH) with moderate to advanced liver fibrosis. |
|
August 2025 |
Madrigal Pharmaceuticals |
Madrigal Pharmaceuticals obtained a new US Patent No. 12,377,104 for Rezdiffra (resmetirom), an FDAapproved therapy for metabolic dysfunctionassociated steatohepatitis (MASH) with moderate to advanced fibrosis. |
|
August 2025 |
Novo Nordisk |
The company received US FDA approval for Wegovy, used to treat adults with moderate to advanced liver fibrosis. |
|
July 2025 |
Bausch Health Companies Inc. |
The company agreed to acquire DURECT Corporation for the novel therapeutic molecule larsucosterol, which demonstrated promising results for alcoholic hepatitis. |
|
July 2025 |
Madrigal Pharmaceuticals |
The company received a USD 350 million initial term loan from Blue Owl Capital to advance the development and commercialization of metabolic dysfunction-associated steatohepatitis (MASH), a leading cause of liver fibrosis and eventual cirrhosis. |
Source: Secondary Research
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 19.90 Billion |
| Market Size in 2026 | USD 21.15 Billion |
| Market Size in 2034 | USD 34.81 Billion |
| CAGR | 6.43% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Treatment Type, By Disease Type, By Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Liver Cirrhosis Treatment Market Segments
By Treatment Type
- Immunosuppressants
- Antibiotics
- Vaccines
- Corticosteroids
- Other Treatment Types
By Disease Type
- Alcoholic Cirrhosis
- Atrophic Cirrhosis
- Biliary Cirrhosis
- Cryptogenic Cirrhosis
By Distribution Channel
- Hospital Pharmacies
- Drug Stores & Retail Pharmacies
- Other Distribution Channel
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Dhanashri Bhapakar
Senior Research Associate
Dhanashri Bhapakar is a Senior Research Associate with 3+ years of experience in the Biotechnology sector. She focuses on tracking innovation trends, R&D breakthroughs, and market opportunities within biopharmaceuticals and life sciences. Dhanashri’s deep industry knowledge enables her to provide precise, data-backed insights that help companies innovate and compete effectively in global biotech markets.










































