Ophthalmic Clinical Trials Market Size, Share & Trends Analysis Report By Phase (Discovery Phase, Preclinical Phase, Clinical Phase), By Therapeutic Modality (Drugs, Devices, Others), By Indication (Glaucoma, Macular Degeneration, Dry Eye Disease, Cataract & refractive, Uveitis, Retinopathy, Others), By Sponsor Type (Pharmaceutical & Biopharma Companies, Medical Device Companies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Ophthalmic Clinical Trials Market Overview
The global ophthalmic clinical trials market size is valued at USD 1.79 billion in 2025 and is estimated to reach USD 3.18 billion by 2034, growing at a CAGR of 6.61% during 2026-2034. The global market observed impressive growth stimulated by increasing inter-regulatory harmonization, which enhanced multinational trial efficiency.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 42.63% in 2025.
- The Asia Pacific region is estimated to grow at the fastest pace, with a CAGR of 8.17% during the forecast period.
- Based on phase, the preclinical phase segment is projected to register the fastest CAGR of 6.96%.
- On the basis of therapeutic modality, the devices segment is demonstrated to grow at the fastest CAGR of 7.27% during the forecast period.
- Based on the Indication, the cataract & refractive segment dominated the market in 2025 with a revenue share of 23.85%.
- By sponsor type, the pharmaceutical & biopharma companies segment dominated the market in 2025
- The U.S. dominates the market, valued at USD 653.16 million in 2024 and reaching USD 693.52 million in 2025.
Table: U.S. Ophthalmic Clinical Trials Market Size (USD Million)
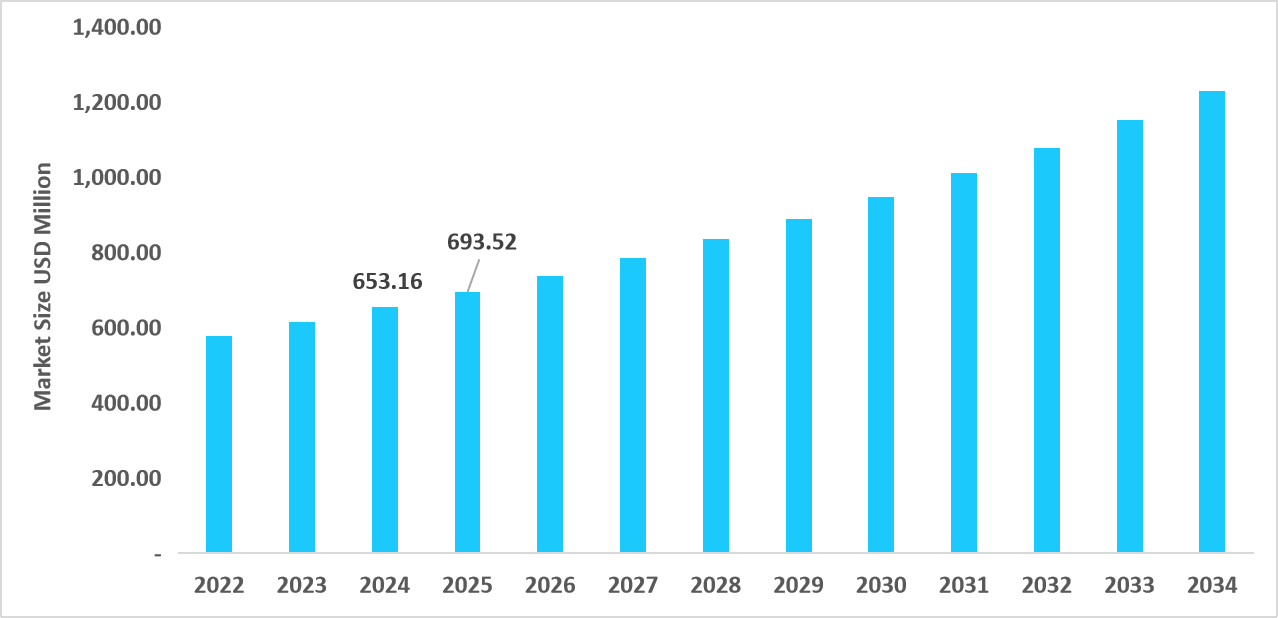
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 1.79 billion
- 2034 Projected Market Size: USD 3.18 billion
- CAGR (2026-2034): 6.61%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The ophthalmic clinical trials market is segmented based on various phases which encompasses discovery, preclinical, and clinical phases, evaluating the safety and efficacy of ophthalmic therapies. The clinical phase is further divided into phase I, phase II, phase II, and phase IV. Therapeutic modalities include drugs, biologics, cell and gene therapies, and medical devices such as surgical, diagnostic, and vision care instruments. These trials address conditions like glaucoma, macular degeneration, dry eye disease, and cataract, sponsored primarily by pharmaceutical, biopharma, and medical device companies.
Latest Market Trends
Emergence of Precision and Personalized Ophthalmology
A major trend in the ophthalmic clinical trials market is the growing shift toward precision and personalized ophthalmology, where treatments are tailored based on genetic, biomarker, and phenotypic data. Companies are developing gene-specific therapies for inherited retinal diseases such as Leber’s congenital amaurosis and retinitis pigmentosa. This precision-based approach enhances therapeutic efficacy and drives the design of more targeted and efficient ophthalmic clinical trials worldwide.
Growing Adoption of Decentralized and Virtual Ophthalmic Clinical Trials
The rising adoption of decentralized and virtual trial models is a key trend for market growth. Through teleophthalmology, remote patient monitoring, and digital imaging tools, these models reduce site visits and enhance patient accessibility. This shift toward digitalization improves efficiency and reduces operational costs, thereby accelerating ophthalmic research and market growth.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 1.79 Billion |
| Estimated 2026 Value | USD 1.90 Billion |
| Projected 2034 Value | USD 3.18 Billion |
| CAGR (2026-2034) | 6.61% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | IQVIA, Apellis Pharmaceuticals, Carl Zeiss Meditec, Charles River Laboratories, Hoffmann-La Roche Ltd |
Ophthalmic Clinical Trials Market Drivers
Rising Prevalence of Diabetes and Associated Ocular Disorders
The growing global incidence of diabetes is a major driver for the global market, as diabetic patients are at higher risk of developing vision-threatening conditions such as diabetic retinopathy and macular edema. According to the International Diabetes Federation (IDF), over 540 million adults worldwide are living with diabetes. This escalating disease burden is fueling demand for innovative ophthalmic treatments, thereby driving increased clinical trial activity focused on diabetic eye disease management.
Market Restraints
High Operational Costs and Recruitment Challenges
A major restraint in the ophthalmic clinical trials market is the high operational cost and difficulty in patient recruitment, for rare ocular diseases. Conducting multicenter trials demand advanced imaging technologies and specialized investigators, which notably increases overall expenses.
According to industry reports, recruitment delays and complex trial designs often extend development timelines, thereby restraining market growth and limiting participation from smaller research organizations.
Market Opportunities
Increasing Clinical Trial Activities for Cell and Gene-Based Therapies
The growing number of clinical studies focused on cell and gene-based therapies for vision restoration is a key opportunity for market growth. Advancements in molecular biology and regenerative medicine have accelerated the development of novel treatments for inherited retinal diseases. The ongoing global trials exploring CRISPR-based gene editing and stem cell-derived retinal implants highlight the increasing investment and potential of these transformative therapeutic approaches in ophthalmology.
Regional Analysis
North America dominated the market in 2025, accounting for 42.63% market share. This growth is primarily driven by the increasing prevalence of age-related ocular disorders such as macular degeneration, glaucoma, and diabetic retinopathy. The aging population led to a surge in vision related diseases, creating a strong demand for innovative ophthalmic treatments and fostering the expansion of clinical research across the region.
Canada's ophthalmic clinical trials market is influenced by the country's robust healthcare infrastructure and provincial reimbursement policies. For instance, the adoption of advanced imaging technologies and the introduction of cost effective biosimilar anti-VEGF agents have expanded patient access to treatments. These developments not only enhance the quality of care but also stimulate the growth of clinical research activities within the ophthalmic sector.
Asia Pacific Market Insights
Asia Pacific is emerging as a fastest growing region with a CAGR of 8.17% from 2026-2034. The growth is driven by the availability of a large, treatment-naive patient population, which enables faster patient recruitment and enrollment. Countries such as India, China, and South Korea offer diverse patient demographics and high disease prevalence, making the region an attractive destination for multicenter ophthalmic trials and accelerating clinical research activities.
Japan ophthalmic clinical trials market is expanding due to the country's pioneering use of induced pluripotent stem cells in regenerative ophthalmology. Japan was the first to approve human clinical trials using autologous iPSCs for retinal diseases, such as age-related macular degeneration. This regulatory support fosters innovation and positions Japan as a global leader in advanced ocular therapies.
Regional Market share (%) in 2025
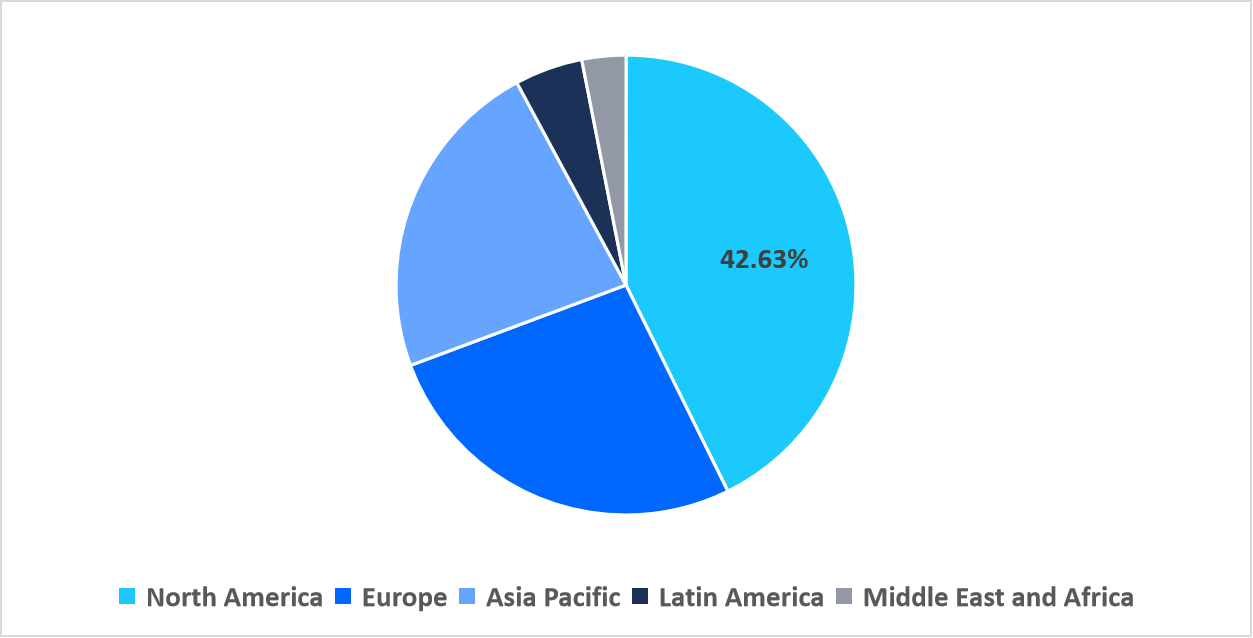
Source: Straits Research
Europe Market Insights
In Europe the market growth is accelerated by the growing focus on rare and genetic ocular diseases. European countries, including Germany, France, and the UK, established specialized research centers and patient registries for inherited retinal disorders. This infrastructure enables efficient recruitment for niche trials and fosters innovation in gene and cell-based ophthalmic therapies across the region.
In UK, the ophthalmic clinical trials market is experiencing growth due to the government supported National Health Service (NHS) Digital Eye Health programs, which provide extensive real world patient data. This rich dataset allows precise patient stratification and accelerates recruitment for rare and complex ocular disease trials, positioning the UK as a preferred hub for innovative ophthalmic research.
Latin America Market Insights
In Latin America, the ophthalmic clinical trials market growth is propelled by the region’s growing adoption of public–private partnerships (PPPs) in healthcare research. Mexico and Chile are increasingly collaborating with international pharmaceutical companies to co-fund ophthalmic trials, enhancing research capacity, sharing expertise, and accelerating the development of innovative ocular therapies across the region.
In Brazil, the ophthalmic clinical trials market growth is augmented by the country’s established network of ophthalmology research centers and specialized eye hospitals. This infrastructure supports rapid patient recruitment and high-quality data collection, making Brazil an attractive destination for multicenter trials and fostering the development of innovative therapies for conditions such as diabetic retinopathy and glaucoma.
Middle East and Africa Market Insights
In the Middle East, the market is expanding due to the rising investment in medical tourism and specialized eye care centers, mainly in countries like the UAE and Saudi Arabia. These initiatives attract international clinical research, enhance access to diverse patient populations, and support the growth of region-specific ophthalmic trials.
In South Africa, the ophthalmic clinical trials market is augmented by the country’s extensive HIV and diabetes patient population, which increases the prevalence of associated ocular complications such as diabetic retinopathy and opportunistic eye infections. This provides an opportunity for targeted ophthalmic research and clinical trials addressing region specific disease burdens.
Phase Insights
The clinical phase segment dominated the market in 2025 with a revenue share of 71.69%. This growth is driven by the increasing number of late stage ophthalmic drug candidates progressing to Phase II and III trials. The rise in advanced pipeline products reflects strong industry confidence, resulting in higher clinical trial activity and revenue generation within this segment.
The preclinical phase segment is projected to witness the fastest CAGR of 6.96% during the forecast timeframe. This growth is augmented by the rapid adoption of advanced in vitro and in vivo ocular models, including 3D retinal organoids and animal models, which enhance early-stage drug screening accuracy and accelerate preclinical evaluation for novel ophthalmic therapies.
Therapeutic Modality Insights
The drugs segment dominated the market in 2025 with a revenue share of 72.19%, owing to the expanding use of biologics and monoclonal antibodies for treating retinal disorders such as wet AMD and diabetic macular edema, leading to increased clinical research investments and higher trial activity within the ophthalmic drugs segment.
The devices segment is expected to register fastest CAGR of 7.27% during the forecast period. This growth is supported by the rising integration of digital ophthalmic devices, such as AI powered imaging systems and portable diagnostic tools, which enhance real-time data collection and patient monitoring, thereby accelerating clinical trial efficiency and technological adoption in ophthalmic research.
By Therapeutic Modality Market Share (%), 2025
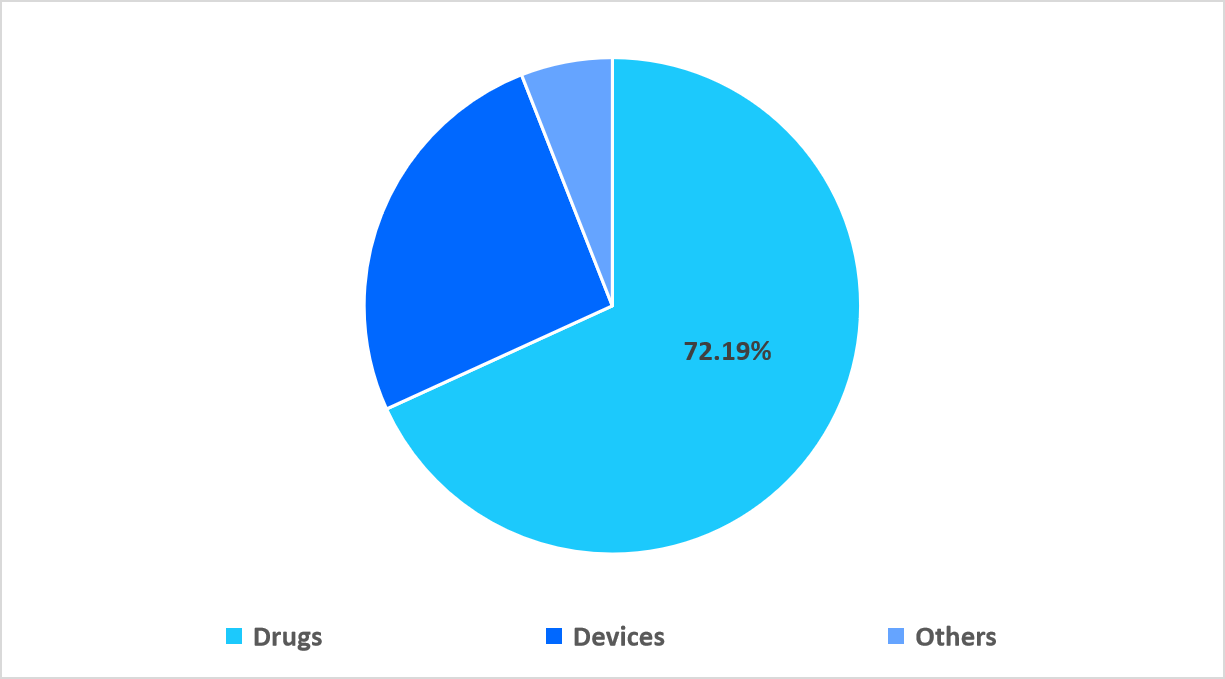
Source: Straits Research
Indication Insights
The cataract & refractive segment dominated the market in 2025. This growth is driven by the increasing adoption of advanced intraocular lens technologies and femtosecond laser-assisted surgeries, which have improved visual outcomes and patient satisfaction, thereby stimulating clinical research and innovation in cataract and refractive treatment studies.
The glaucoma segment is estimated to grow at a CAGR of 7.46% during the forecast period. This growth is stimulated by the growing emphasis on neuroprotective and pressure-independent therapies targeting optic nerve preservation in glaucoma patients. Such novel treatment approaches are encouraging extensive clinical research and driving innovation in next-generation glaucoma drug development.
Sponsor Type Insights
The pharmaceutical & biopharma companies segment dominated the market in 2025, accounting for 42.69% revenue share in 2025, owing to the increasing strategic collaborations between pharmaceutical and biopharma companies to co-develop novel ophthalmic therapies, leveraging shared expertise, funding, and technology platforms to accelerate clinical development and expand their global ophthalmic research pipelines.
Competitive Landscape
The global ophthalmic drugs market is moderately consolidated, with a few major players dominating overall revenue share. Ongoing advancements in drug delivery systems, such as sustained release formulations and gene therapies, have intensified competition. Key global players in the market include Novartis AG, Roche Holding AG, Regeneron Pharmaceuticals, Bayer AG, AbbVie Inc., and others. These companies are actively engaging in strategic collaborations, mergers, and product launches to strengthen their portfolios and sustain a competitive position within the global ophthalmic drugs industry.
Clearside Biomedical: An emerging market player
Clearside Biomedical is an emerging player in the ophthalmic clinical trials market and is a clinical stage biopharmaceutical company focused on developing innovative therapies for retinal and choroidal diseases. The company utilizes its proprietary suprachoroidal space delivery platform to enhance targeted drug delivery and improve treatment outcomes.
- For instance, in March 2024, Clearside Biomedical initiated a multicenter Phase II clinical study across the U.S. and Europe to evaluate the safety and efficacy of CLS-AX for patients with neovascular age-related macular degeneration.
List of Key and Emerging Players in Ophthalmic Clinical Trials Market
- IQVIA
- Apellis Pharmaceuticals
- Carl Zeiss Meditec
- Charles River Laboratories
- Hoffmann-La Roche Ltd
- GenSight Biologics
- ICON plc
- Iris Pharma
- Johnson & Johnson Services Inc.
- Laboratory Corporation of America Holdings
- Medpace
- Novartis AG
- Novotech
- Parexel
- ProTrials Research, Inc.
- Regeneron Pharmaceuticals
- Santen Pharmaceutical
- Syneos Health
- TFS HealthScience
- Others
Strategic Initiatives
- October 2025: Glaukos Corporation announced the U.S. Food and Drug Administration (FDA) approved its Epioxa HD / Epioxa New Drug Application (NDA).
- June 2025: Amneal Pharmaceuticals, Inc. announced the U.S. Food and Drug Administration (FDA) approval of prednisolone acetate ophthalmic suspension, 1% sterile, which references Pred Forte.
- February 2025: Roche received the U.S. FDA approval for Susvimo 100 mg/mL for the treatment of diabetic macular edema (DME).
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 1.79 Billion |
| Market Size in 2026 | USD 1.90 Billion |
| Market Size in 2034 | USD 3.18 Billion |
| CAGR | 6.61% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Phase, By Therapeutic Modality, By Indication, By Sponsor Type |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Ophthalmic Clinical Trials Market Segments
By Phase
- Discovery Phase
- Preclinical Phase
-
Clinical Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Therapeutic Modality
-
Drugs
- Small molecules
- Biologics
- Cell & Gene Therapies
-
Devices
- Surgical & Diagnostics Devices
- Vision care Devices
- Others
By Indication
- Glaucoma
- Macular Degeneration
- Dry Eye Disease
- Cataract & refractive
- Uveitis
- Retinopathy
- Others
By Sponsor Type
- Pharmaceutical & Biopharma Companies
- Medical Device Companies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































