Orphan Drug Market Size, Share & Trends Analysis Report By Drug Type (Biologicals, Non-Biologicals), By Therapy Type (Oncology, Haematology, Neurology, Infectious Diseases, Metabolic Disorders, Endocrinology, Immunology, Others), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Orphan Drug Market Overview
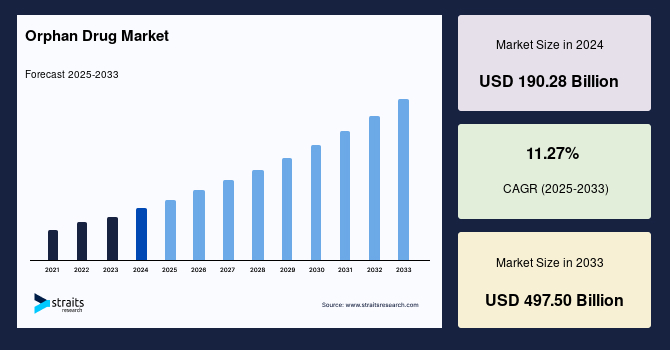
The global orphan drug market size was valued at USD 190.28 billion in 2024 and is projected to grow from USD 211.72 billion in 2025 to reach USD 497.50 billion by 2033, growing at a CAGR of 11.27% during the forecast period (2025–2033). The increasing prevalence of rare and genetic disorders worldwide is fueling the demand for these drugs, as patients require specialized, targeted therapies to manage conditions with limited treatment options.
Key Market Insights
- North America dominates the global orphan drug industry with a 51.87% share, driven by strong regulatory support, advanced healthcare infrastructure, and rapid adoption of gene and cell therapies.
- By therapy type, the oncology segment held the largest share of approximately 37.13% of the orphan drug market in 2024, owing to the high prevalence of rare cancers and the urgent need for targeted therapies.
- By drug type, the biologicals segment is witnessing steady growth, driven by its effectiveness in treating complex and rare diseases.
- By distribution channel, the hospital pharmacies segment dominated the orphan drug industry in 2024 due to their ability to provide specialized care and manage complex therapies.
Market Size & Forecast
- 2024 Market Size: USD 190.28 billion
- 2033 Projected Market Size: USD 497.50 billion
- CAGR (2025-2033): 11.27%
- North America: Largest market in 2024
- Asia-Pacific: Fastest-growing region
The global orphan drug market is being significantly propelled by increasing awareness among healthcare professionals and patients regarding rare diseases, which facilitates early diagnosis and timely treatment. High unmet medical needs for conditions with limited or no existing therapies are encouraging pharmaceutical and biotech companies to innovate and develop targeted orphan therapeutics.
Additionally, advancements in biotechnology, including gene therapy, cell therapy, and personalized medicine, are enabling the creation of highly effective treatments for rare disorders, driving market growth. Growing investment in research and development, coupled with collaborations between small biotech firms and large pharmaceutical companies, further accelerates the introduction of novel orphan drugs, catering to niche patient populations and expanding market opportunities.
Orphan Drug Market Trends
Strategic Initiatives by the Key Players
A significant trend in the global market is the strategic expansion initiatives undertaken by key players, including mergers, acquisitions, and partnerships. Companies are actively strengthening their rare disease portfolios to accelerate the development of innovative therapies and gain competitive advantages in niche markets. These strategic moves also help optimize R&D pipelines, expand geographic presence, and enhance access to novel treatments for patients.
- A notable example is BioMarin's $270 million acquisition of Inozyme Pharma in May 2025. Inozyme is developing an enzyme replacement therapy for ENPP1 deficiency, a rare genetic condition affecting bones and blood vessels. This acquisition bolsters BioMarin's position in the rare disease space, reflecting a broader industry trend of leveraging strategic initiatives to address high unmet medical needs and advance specialized therapeutic solutions.
Thus, such strategic activities are expected to continue driving innovation and market growth.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 190.28 Billion |
| Estimated 2025 Value | USD 211.72 Billion |
| Projected 2033 Value | USD 497.50 Billion |
| CAGR (2025-2033) | 11.27% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
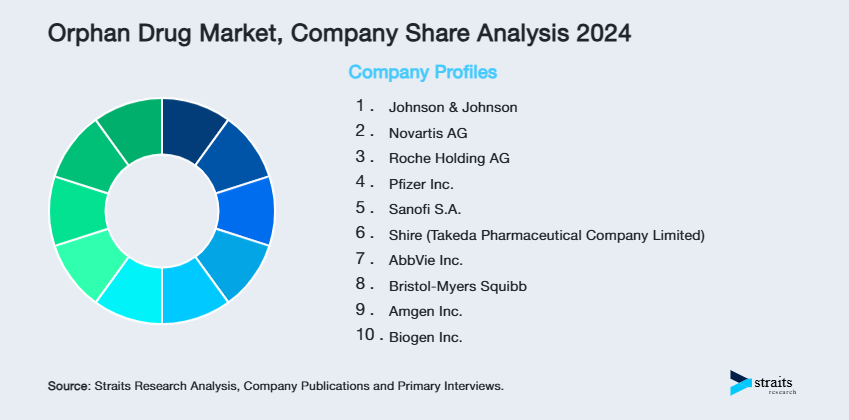
| Key Market Players | Johnson & Johnson, Novartis AG, Roche Holding AG, Pfizer Inc., Sanofi S.A. |
to learn more about this report Download Free Sample Report
Orphan Drug Market Driver
Rising Prevalence of Rare and Genetic Disorders
The rising prevalence of rare and genetic disorders is a prominent driver of the global market. Increasing awareness and improved diagnostic capabilities have led to the identification of more patients affected by rare conditions, creating a growing demand for targeted therapies.
- According to the Orphanet Journal of Rare Diseases, as of 2024, approximately 300 million individuals globally are affected by rare diseases, with an estimated 80% of these conditions having a genetic origin. This collective prevalence translates to about 3.5% to 5.9% of the global population. The high unmet medical need among these patients encourages pharmaceutical firms to invest in research and development of orphan drugs.
Consequently, the market is witnessing a surge in innovative therapies addressing specific rare and genetic disorders, fostering overall growth.
Market Restraint
High Cost
The high cost of orphan drugs is a significant restraint for the global market. Due to the small patient populations for rare diseases, research and development expenses are spread over fewer sales, leading to higher prices for these therapies.
Additionally, the complexity of developing treatments for rare and often genetic disorders adds to manufacturing and clinical trial costs. As a result, patients and healthcare systems face affordability challenges, which can limit access and slow market adoption. Reimbursement issues in several regions further exacerbate the problem, as insurers may be reluctant to cover high-cost therapies, restricting widespread use and overall market growth.
Market Opportunity
Adoption of Regulatory Incentives
Regulatory incentives present a significant market opportunity by reducing development risks and encouraging investment in rare disease therapies. The U.S. Orphan Drug Act of 1983 provides vital support, including a seven-year market exclusivity period, tax credits covering 50% of clinical trial costs, and exemption from FDA user fees. To further encourage development for ultra-rare diseases, the FDA introduced an "ultra-orphan" designation, extending market exclusivity to ten years and offering additional R&D grants.
- A notable example is Soligenix's SuVax vaccine, which received orphan drug designation in April 2024 for Sudan ebolavirus. This designation granted the company access to government grants, waived FDA fees, and financial incentives, resulting in a 52% surge in stock value.
Such incentives encourage pharmaceutical and biotech companies to explore niche rare disease markets, creating growth potential and addressing unmet medical needs.
Regional Analysis
The North American market is witnessing robust growth due to strong regulatory support and a well-established healthcare infrastructure. Accelerated approval pathways and financial incentives are encouraging pharmaceutical and biotech companies to invest heavily in rare disease therapeutics. High patient awareness and advanced diagnostic capabilities are enabling early detection and treatment of rare disorders. Additionally, collaborations between research institutions and industry players are driving innovation in gene and cell therapies, further strengthening the market's expansion and adoption of cutting-edge orphan drugs.
U.s. Orphan Drug Market Trends
- The U.S. market is expanding rapidly, supported by the Orphan Drug Act and FDA incentives like market exclusivity and tax credits. Major players such as Pfizer and Biogen are investing heavily in rare disease treatments, including gene therapies and biologics. Growing patient advocacy and advanced diagnostics are improving early detection and treatment, fueling the market's growth in the country.
- Canada's orphan drug industry is witnessing growth due to the surging prevalence of rare diseases and supportive government policies. Health Canada's priority review pathway and access programs facilitate faster approvals and patient access. Collaborations between Canadian biotech firms and global pharmaceutical companies, such as Apotex's partnerships in rare disease therapies, are enhancing the orphan drug pipeline. Rising awareness and diagnostic advancements are further driving market demand.
Asia-Pacific Significantly Growing Region
The Asia-Pacific market is expanding rapidly due to improving healthcare infrastructure and growing investment in rare disease research. Increased awareness among healthcare professionals and patients is driving early diagnosis and treatment adoption. Supportive policies and incentives are encouraging local and international companies to enter the market. Additionally, rising collaborations between biotech firms and healthcare providers are enhancing the availability of advanced therapies. The region's large population base presents significant growth potential for these drugs, especially in the adoption of innovative therapies for previously untreated rare conditions.
- China's orphan drug market is rapidly expanding due to increasing government support and favorable policies, such as the National Rare Disease List and fast-track approvals. Rising awareness of rare diseases and growing R&D investments by local firms like Shanghai Pharma and Hansoh Pharma are driving growth. Additionally, collaborations with global biotech companies are accelerating the development and availability of orphan therapies across the country.
- India's market for orphan drug is witnessing steady growth, driven by rising rare disease awareness and supportive initiatives from the government, including the National Policy for Rare Diseases. Domestic companies like Biocon and Intas Pharmaceuticals are investing in research and development of orphan drugs. Partnerships with international pharmaceutical firms are improving access to advanced therapies, while increasing healthcare infrastructure supports wider distribution in both urban and rural regions.
Europe Substantial Potential for Growth
Europe's market is growing steadily, supported by favorable government policies and orphan drug legislation that provide market exclusivity and financial incentives. The surging prevalence of rare diseases and rising patient awareness are boosting demand. Strong research networks and clinical trial infrastructure facilitate the development of innovative therapies. Cross-border collaborations between pharmaceutical companies and healthcare providers improve patient access to orphan drugs. The area is also witnessing growing adoption of advanced therapies, including biologics and gene-based treatments, which drive market growth and address unmet medical needs.
- Germany's market is witnessing significant growth due to strong government support and a well-established healthcare infrastructure. Incentives such as early access programs and reimbursement policies encourage pharmaceutical companies to develop orphan therapies. Companies like BioNTech and Bayer are actively investing in rare disease treatments. Additionally, the surging awareness among healthcare professionals and patients drives early diagnosis and adoption of orphan drugs.
- The UK's orphan drug market is expanding, supported by the National Health Service (NHS) and favorable regulatory policies from the Medicines and Healthcare products Regulatory Agency (MHRA). Initiatives like the UK Rare Diseases Framework promote research and access to rare disease therapies. Pharmaceutical companies, including GlaxoSmithKline and AstraZeneca, are investing in orphan drug development, focusing on gene therapies and targeted treatments for rare genetic disorders.
Market Segmentation
Drug Type Insights
The biologicals segment dominates the global market due to its effectiveness in treating complex and rare diseases. These drugs, including monoclonal antibodies, recombinant proteins, and gene therapies, offer targeted treatment options that address unmet medical needs. Advancements in biotechnology and personalized medicine have further accelerated the development of biological orphan drugs. Their ability to provide long-term therapeutic benefits and improved patient outcomes makes them a preferred choice among healthcare providers. Additionally, strong R&D investments and supportive regulatory frameworks enhance the growth and adoption of biologicals in the global market.
Therapy Type Insights
Oncology holds a dominant position in the market, driven by the high prevalence of rare cancers and the urgent need for targeted therapies. These drugs for oncology offer specialized treatment options, including immunotherapies and targeted therapies, addressing unmet medical needs in rare cancer types. Continuous advancements in cancer research, precision medicine, and biomarker identification have fueled the development of novel oncological orphan drugs. Strong investment from pharmaceutical and biotechnology companies and favorable regulatory incentives further support growth. The rising awareness among patients and healthcare providers about rare cancers contributes to the oncology segment's leadership in the market.
Distribution Channel Insights
Hospital pharmacies lead the distribution channel in this market due to their ability to provide specialized care and manage complex therapies. Patients with rare diseases often require treatments that need careful monitoring, administration, and dosage adjustments, which hospital pharmacies are equipped to handle. Additionally, hospitals collaborate closely with healthcare providers to ensure proper storage, handling, and timely delivery of these drugs. The presence of trained staff and infrastructure to manage high-cost and sensitive medications makes hospital pharmacies the primary distribution channel. Furthermore, partnerships between hospitals and pharmaceutical companies facilitate access to orphan drugs, supporting the hospital pharmacies segment's dominance in the market.
Company Market Share
Leading companies in the global market are focusing on expanding their pipelines for rare and genetic disorders through innovative research and development. They are investing in advanced therapies like gene and cell therapies, forming strategic collaborations, and pursuing acquisitions to enhance their portfolios. Additionally, companies are leveraging regulatory incentives, accelerating clinical trials, and strengthening patient support programs to improve market access and drive growth in underserved rare disease segments.
Novartis AG, a Swiss multinational pharmaceutical company, is a prominent player in the global market. The company focuses on developing innovative therapies for rare and genetic diseases through its Sandoz and Novartis Pharmaceuticals divisions. Novartis has a strong orphan drug portfolio, including treatments for conditions like spinal muscular atrophy, sickle cell disease, and rare cancers. Leveraging advanced technologies like gene therapy and biologics, Novartis actively invests in research collaborations and regulatory incentives to expand access and address unmet medical needs worldwide.
- In June 2025, ianalumab, an investigational therapy from Novartis, received orphan drug designation from the U.S. FDA for the treatment of immune thrombocytopenia (ITP). The Phase III trial demonstrated statistically significant improvement in time to treatment failure.
List of Key and Emerging Players in Orphan Drug Market
- Johnson & Johnson
- Novartis AG
- Roche Holding AG
- Pfizer Inc.
- Sanofi S.A.
- Shire (Takeda Pharmaceutical Company Limited)
- AbbVie Inc.
- Bristol-Myers Squibb
- Amgen Inc.
- Biogen Inc.
to learn more about this report Download Market Share
Recent Developments
- August 2025- Brensocatib was approved by the U.S. FDA for treating non-cystic fibrosis bronchiectasis, a rare chronic lung condition. This approval introduces a novel therapeutic option, addressing significant unmet medical needs. The drug’s launch strengthens treatment accessibility for patients and underscores the growing focus on developing effective therapies for rare respiratory diseases.
- July 2025- The U.S. FDA approved Sephience™ (sepiapterin) by PTC Therapeutics for treating phenylketonuria (PKU) in patients aged 1 month and older. This oral therapy enhances the activity and stability of the phenylalanine hydroxylase enzyme, effectively reducing blood phenylalanine levels by an average of 63%. Clinical trials demonstrated that over 97% of participants could liberalize their diet while maintaining controlled phenylalanine levels.
- June 2025- the U.S. FDA granted orphan drug designation to Sanofi’s rilzabrutinib, an oral Bruton's tyrosine kinase (BTK) inhibitor, for treating sickle cell disease, a rare blood disorder. This designation supports expedited development and regulatory incentives, highlighting Sanofi’s focus on innovative therapies for rare conditions with high unmet medical needs in the market.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 190.28 Billion |
| Market Size in 2025 | USD 211.72 Billion |
| Market Size in 2033 | USD 497.50 Billion |
| CAGR | 11.27% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Drug Type, By Therapy Type, By Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Orphan Drug Market Segments
By Drug Type
- Biologicals
- Non-Biologicals
By Therapy Type
- Oncology
- Haematology
- Neurology
- Infectious Diseases
- Metabolic Disorders
- Endocrinology
- Immunology
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Mitiksha Koul
Research Associate
Mitiksha Koul is a Research Associate with 2 years of experience in market research. She focuses on analyzing industry trends, competitive landscapes, and growth opportunities to support strategic decision-making. Mitiksha’s strong analytical skills and research expertise enable her to deliver actionable insights that help businesses adapt to evolving market dynamics and achieve sustainable growth.










































