Peptide Antibiotics Market Size, Share & Trends Analysis Report By Type (Ribosomal Synthesized Peptide Antibiotics, Non-ribosomal Synthesized Peptide Antibiotics), By Route of Administration (Parenteral, Oral, Topical), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Peptide Antibiotics Market Size
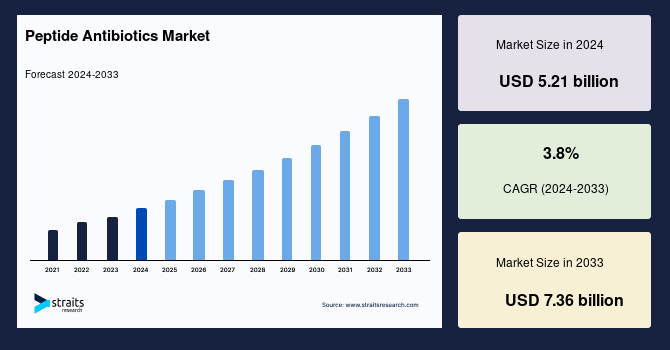
The global peptide antibiotics market size was valued at USD 5.21 Billion in 2024 and is anticipated to grow from USD 5.41 Billion in 2025 to reach USD 7.29 Billion by 2033, exhibiting a CAGR of 3.8% during the forecast period (2025–2033).
Peptide antibiotics are a class of antibiotics made up of short chains of amino acids, known as peptides, that exhibit antimicrobial activity. These antibiotics work by targeting bacterial cell membranes or interfering with key bacterial processes, ultimately inhibiting the growth of or killing harmful bacteria. These are typically used to treat infections caused by drug-resistant bacteria, as they often remain effective against a wide range of pathogens that are resistant to traditional antibiotics.
Some well-known peptide antibiotics include polymyxins (such as polymyxin B and colistin) and bacitracin. These antibiotics are commonly used in situations where other antibiotics may no longer be effective due to the growing issue of antimicrobial resistance. The market is experiencing substantial growth, fueled by the rising threat of antimicrobial resistance (AMR), the growing demand for novel antibiotics, and significant advancements in peptide engineering.
The prevalence of multidrug-resistant (MDR) infections, particularly in hospital environments, is driving the need for innovative peptide-based therapies that offer greater efficacy and lower risks of resistance. This surge in demand is further supported by increased investments from pharmaceutical companies, government initiatives, and funding organizations such as CARB-X, all of which are accelerating research and development in this sector.
Technological advancements, such as AI-driven drug discovery, synthetic biology, and cutting-edge formulation techniques, are unlocking new opportunities for market growth. Additionally, strategic collaborations between biotech firms and contract manufacturing organizations (CMOs) enhance peptide antibiotic production's scalability. As healthcare infrastructure improves and the demand for antibiotics rises, especially in emerging economies, the market is positioned for robust expansion.
Market Trends
Advancements in Synthetic Peptide Engineering
Advancements in synthetic peptide engineering, including AI-driven design and structural modifications, enhance the stability, potency, and bioavailability of these antibiotics. Technologies like solid-phase synthesis and recombinant DNA methods improve resistance to enzymatic degradation and reduce toxicity, making them more effective against multidrug-resistant bacteria.
- In January 2025, a study published by the National Library of Medicine highlighted the use of AI and machine learning in designing antimicrobial peptides (AMPs). These technologies enhance peptide stability, reduce toxicity, and accelerate drug discovery. AI-driven models predict peptide properties, addressing key challenges in traditional antibiotic development and strengthening efforts against antimicrobial resistance.
The study highlights the potential of data-driven approaches to overcome current limitations and expedite the clinical translation of effective.
Rising Clinical Trials & Regulatory Approvals
The increasing prevalence of multidrug-resistant (MDR) infections has increased the clinical trials and regulatory approvals for peptide antibiotics. Governments and regulatory bodies such as the FDA and EMA are granting fast-track and priority review designations to novel antimicrobial peptides (AMPs).
- For instance, in February 2025, AbbVie announced that the U.S. Food and Drug Administration (FDA) approved EMBLAVEO (aztreonam and avibactam), the first and only fixed-dose intravenous monobactam/β-lactamase inhibitor combination antibiotic. It is approved for use with metronidazole in patients aged 18 and older who have limited or no alternative options for treating complicated intra-abdominal infections (cIAI).
Such approval highlights the growing regulatory support for innovative peptide-based antibiotics, boosting the global market.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 5.21 Billion |
| Estimated 2025 Value | USD 5.41 Billion |
| Projected 2033 Value | USD 7.29 Billion |
| CAGR (2025-2033) | 3.8% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
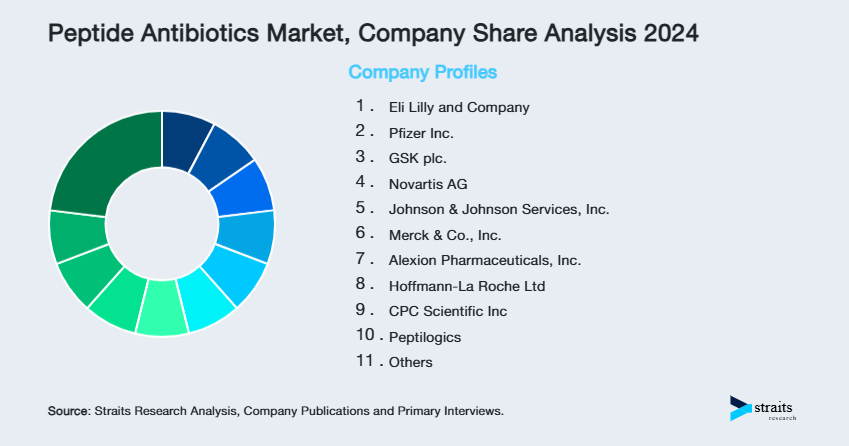
| Key Market Players | Eli Lilly and Company, Pfizer Inc., GSK plc., Novartis AG, Johnson & Johnson Services, Inc. |
to learn more about this report Download Free Sample Report
Peptide Antibiotics Market Growth Factors
Rising Hospital-Acquired Infections (hais)
The increasing incidence of hospital-acquired infections (HAIs), such as bloodstream infections (BSIs), ventilator-associated pneumonia (VAP), and surgical site infections (SSIs), is driving demand. Multidrug-resistant (MDR) pathogens such as Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa are responsible for severe HAIs, often resistant to conventional antibiotics.
- For instance, in November 2024, the Centre for Disease Control and Prevention (CDC) estimated that HAIs affect 1 in 31 hospitalized patients daily in the U.S., contributing to rising healthcare costs and increased mortality rates.
Such a growing burden highlights the urgent need for effective peptide antibiotics, which offer targeted solutions against MDR pathogens, which is driving the market growth.
Growing Investments in Antimicrobial Research
Increasing global investments in antimicrobial research are driving the development of peptide antibiotics. Governments, pharmaceutical companies, and organizations such as Combating Antibiotic Resistant Bacteria (CARB-X) and the AMR Action Fund are funding innovative antimicrobial peptide (AMP) research to combat multidrug-resistant (MDR) bacteria.
- For instance, in January 2025, CARB-X awarded a $610K seed grant to Justus Liebig University Giessen (JLU) to develop a first-in-class peptide antibiotic targeting the BamA protein in Gram-negative bacteria. This innovative approach aims to treat infections such as complicated urinary tract infections (UTIs) and cystic fibrosis-related lung infections.
Such investments highlight the growing commitment to advancing peptide antibiotics, accelerating the development of novel treatments to combat MDR infections and address critical global health challenges.
Market Restraining Factors
Limited Oral Bioavailability
One of the key restraining factors in the global peptide antibiotics market is their limited oral bioavailability. Most of these antibiotics are susceptible to degradation in the gastrointestinal tract, preventing them from reaching systemic circulation when taken orally. As a result, intravenous or other non-oral delivery methods are required, which can reduce patient compliance and limit widespread use.
While advancements in nanoparticle formulations, enzyme inhibitors, and peptide modifications are being explored to enhance oral stability and absorption, challenges persist in achieving effective bioavailability without compromising therapeutic efficacy. These limitations hinder the market's potential for broader application and accessibility.
Market Opportunity
Advancements in Customized Orthodontic Treatments
Advancements in drug delivery technologies present a significant opportunity for peptide antibiotics. Researchers are developing novel formulations such as nanoparticle-based delivery, prodrug approaches, and peptide modifications to enhance stability, bioavailability, and targeted drug release.
- For instance, in November 2024, researchers developed a gut-targeted engineered particle vaccine (EPV) delivery system to enhance the bioavailability of hybrid antimicrobial peptides (HAMPs) against Clostridium perfringens This innovative approach improved peptide stability and specificity, showing significant antimicrobial activity in preclinical studies.
Such advancements boost market growth by enabling next-generation antibiotics with improved efficacy, broader applications, and better patient compliance.
Regional Insights
North America holds a dominant position in the global peptide antibiotics market, largely due to its robust research and development ecosystem and a high rate of regulatory approvals for novel antimicrobial therapies. The rising prevalence of multidrug-resistant (MDR) infections, coupled with government initiatives like CARB-X and NIH funding, significantly contribute to market expansion.
The presence of major pharmaceutical companies such as Pfizer, Merck & Co., and others, who are actively investing in the development of peptide antibiotics, further strengthens the region’s market leadership. These key players are pivotal in advancing innovation and driving the adoption of peptide-based therapies, ensuring North America remains a leader.
U.s. Peptide Antibiotics Market Trends
The U.S. market is leading, benefiting from significant investments in research and development and the expansion of facilities by key industry players. In June 2022, CPC Scientific Inc. opened a new peptide API manufacturing facility in California, enhancing clinical-to-commercial peptide production. This expansion boosts supply chain capabilities and creates job opportunities, solidifying the U.S. position as a global hub for peptide antibiotic innovation.
Asia-Pacific is poised to register the highest CAGR in the market during the forecast period. This rapid growth is driven by the increasing burden of antimicrobial resistance (AMR), rising healthcare investments, and expanding pharmaceutical manufacturing capabilities in the region. Countries like China, India, and Japan are witnessing a surge in clinical trials and regulatory approvals for peptide-based antimicrobial therapies. Moreover, government initiatives focused on antibiotic stewardship and the growing number of biotech startups specializing in antimicrobial peptides are accelerating market growth.
India's peptide antibiotics market is rapidly growing, driven by rising AMR, expanding government initiatives, and robust pharmaceutical manufacturing capabilities. Organizations like the Indian Council of Medical Research (ICMR) and the Department of Biotechnology (DBT) are funding AMR research and antibiotic development. Moreover, India’s strong generic drug industry and growing biotech sector are facilitating innovation in peptide-based antibiotics, making it a key player.
China’s peptide antibiotics industry is being propelled by rising AMR and increased government investments in biotechnology. The Chinese government’s support, particularly through initiatives such as the National Science and Technology Major Project for Drug Innovation, is advancing research in antimicrobial peptides. China's rapidly expanding pharmaceutical manufacturing sector is also positioning the country as a leader in peptide antibiotics, contributing to global efforts to tackle antimicrobial resistance.
Japan is witnessing significant growth in the market, driven by innovative research and development. In February 2023, researchers at Hokkaido University developed a new method for designing and producing peptide antibiotics in large quantities, marking a critical step in combating antibiotic resistance. Japan’s ongoing advancements in peptide antibiotic production, backed by strong research initiatives, continue to drive growth in the country’s peptide antibiotics industry.
Europe Peptide Antibiotics Market Trends
Germany is one of the largest markets for peptide antibiotics in Europe, driven by advanced treatment options and the expansion of new facilities. For instance, in November 2024, CordenPharma expanded its facility in Germany to strengthen peptide development and small-scale manufacturing, including peptide antibiotics. This expansion supports Phase I and II clinical programs with GMP manufacturing suites.
The UK’s peptide antibiotics market is expanding due to the growing threat of antimicrobial resistance (AMR) and strong government support for novel antibiotic research. The National Institute for Health and Care Research (NIHR) and Innovate UK fund research into antimicrobial peptides (AMPs). This commitment to innovative antimicrobial therapies, coupled with regulatory support, positions the UK as a key player in the development of peptide antibiotics.
Canada's market for peptide antibiotics is experiencing growth due to rising AMR concerns, increased government funding, and expanding biopharmaceutical research. The Canadian Institutes of Health Research (CIHR) and the National Research Council (NRC) are key players in supporting novel antibiotic development. Their investments, coupled with strong research initiatives, help strengthen Canada’s position in the global market, driving innovation in the sector.
Type Insights
The Ribosomal Synthesized segment leads the market, driven by their high efficiency in targeting bacterial infections, lower toxicity, and broad-spectrum antimicrobial activity. Naturally produced peptides, such as bacteriocins, are particularly effective against multidrug-resistant (MDR) pathogens, making them an attractive option for clinical use. Their ability to address emerging resistance challenges is fueling their widespread adoption, particularly in hospital settings where MDR infections are more prevalent.
Route of Administration Insights
The parenteral route dominates the market, primarily due to the limited oral bioavailability of most peptide antibiotics. To ensure effective systemic absorption, intravenous or intramuscular administration is essential. This route allows for faster action, higher potency, and improved therapeutic outcomes, particularly when treating severe infections caused by multidrug-resistant (MDR) pathogens. Parenteral administration is especially critical in hospital environments, where timely and efficient treatment is necessary for patient recovery.
Distribution Channel Insights
Hospital pharmacies hold the largest market share, driven by the fact that these antibiotics are predominantly used in inpatient settings for treating severe infections. Hospitals are equipped with specialized infrastructure to properly administer peptide-based therapies, ensuring accurate dosage, storage, and handling. This controlled environment supports the effective use of these medications, leading to their dominance in the market. The critical nature of infections treated in hospitals further reinforces the reliance on hospital pharmacies.
Company Market Share
Key players in the peptide antibiotics industry are increasingly adopting a range of strategic business initiatives to strengthen their market position. These strategies include forging strategic collaborations with research institutions and pharmaceutical companies, securing product approvals to expand their portfolio, pursuing acquisitions to enhance capabilities, and launching innovative new products to meet the growing demand.
Cordenpharma: An Emerging Player in the Global Peptide Antibiotics Market
CordenPharma is a leading Contract Development and Manufacturing Organization (CDMO) specializing in the development and manufacturing of peptide-based therapeutics, including peptide antibiotics. With its state-of-the-art GMP facilities and deep expertise in large-scale peptide synthesis, the company provides end-to-end solutions for pharmaceutical clients, covering everything from early-stage development to commercial production.
Recent Developments by Cordenpharma:
- In January 2023, CordenPharma signed a multi-year agreement for the contract manufacturing of a large-volume peptide at its CordenPharma Colorado facility, reinforcing its commitment to scaling peptide antibiotic production and supporting global pharmaceutical demand.
List of Key and Emerging Players in Peptide Antibiotics Market
- Eli Lilly and Company
- Pfizer Inc.
- GSK plc.
- Novartis AG
- Johnson & Johnson Services, Inc.
- Merck & Co., Inc.
- Alexion Pharmaceuticals, Inc.
- Hoffmann-La Roche Ltd
- CPC Scientific Inc
- Peptilogics
- AMP Biotech
- Phoenix Biotechnology Inc.
- Novabiotics
- Flagship
- CordenPharma
to learn more about this report Download Market Share
Recent Developments
- January 2025 – CARB-X awarded Peptilogics $3.3 million to develop a slow-release formulation of zaloganan-CR, a novel broad-spectrum engineered peptide. Designed to prevent infections following high-energy traumatic bone injuries, these innovative therapeutic aims to enhance post-injury care and combat antimicrobial resistance.
Analyst Opinion
As per our analyst, the market is poised for substantial growth, fueled by the escalating prevalence of antimicrobial resistance (AMR) and increased investment in novel antibiotic research. Technological innovations, such as AI-driven peptide design and advanced drug delivery systems, are accelerating drug discovery, optimizing therapeutic efficacy, and opening new avenues for treatment. Furthermore, regulatory support, including fast-track approvals for promising therapies, is creating a favorable environment for market expansion.
However, the global peptide antibiotics market faces challenges, such as the limited oral bioavailability of many peptide antibiotics, which necessitates alternative delivery methods like intravenous or intramuscular administration. Despite this, ongoing advancements in nanoparticle formulations, peptide modifications, and enzyme inhibitors are offering solutions to enhance oral stability and absorption.
Emerging markets, especially in the Asia-Pacific region, offer significant growth opportunities. Moreover, rising healthcare investments, increasing hospital-acquired infections (HAIs), and expanding pharmaceutical research and development infrastructure in these regions are driving the demand for peptide-based therapies.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 5.21 Billion |
| Market Size in 2025 | USD 5.41 Billion |
| Market Size in 2033 | USD 7.29 Billion |
| CAGR | 3.8% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Route of Administration, By Distribution Channel |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Peptide Antibiotics Market Segments
By Type
- Ribosomal Synthesized Peptide Antibiotics
- Non-ribosomal Synthesized Peptide Antibiotics
By Route of Administration
- Parenteral
- Oral
- Topical
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Mitiksha Koul
Research Associate
Mitiksha Koul is a Research Associate with 2 years of experience in market research. She focuses on analyzing industry trends, competitive landscapes, and growth opportunities to support strategic decision-making. Mitiksha’s strong analytical skills and research expertise enable her to deliver actionable insights that help businesses adapt to evolving market dynamics and achieve sustainable growth.










































