Protein Therapeutics Market Size, Share & Trends Analysis Report By Product (Monoclonal Antibodies, Insulin, Fusion Protein, Erythropoietin, Interferon, Human Growth Hormone, Follicle Stimulating Hormone), By Applications (Metabolic Disorders, Immunologic Disorders, Haematological Disorders, Cancer, Genetic Disorders, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Protein Therapeutics Market Overview
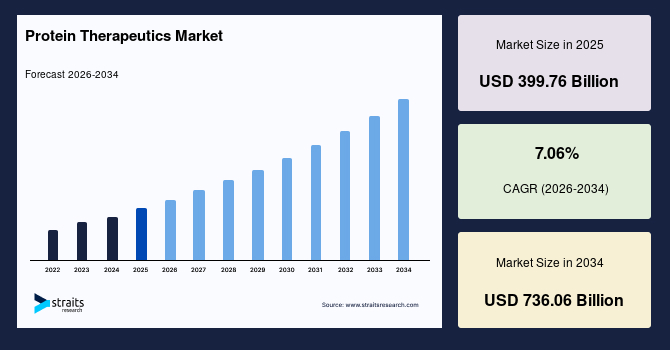
The global protein therapeutics market size is estimated at USD 399.76 billion in 2025 and is projected to reach USD 736.06 billion by 2034, growing at a CAGR of 7.06% from 2026-2034. Strong growth of the market is due to the rising prevalence of chronic diseases such as cancer, diabetes, and autoimmune disorders, coupled with the increasing adoption of biologics over conventional drugs.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 46.92% share in 2025.
- The Asia Pacific region is growing at the fastest pace, with a CAGR of 9.57%, due to several factors.
- Product: The monoclonal antibodies dominated the market in 2025 with a revenue share of 37.21%.
- The Application: Immunologic disorders segment is expected to register the fastest CAGR growth of 8.93%.
- The U.S. dominates the global market, valued at USD 159.94 billion in 2024 and reaching USD 170.54 billion in 2025.
Graph: U.S. Market Revenue Forecast (2022 – 2034)
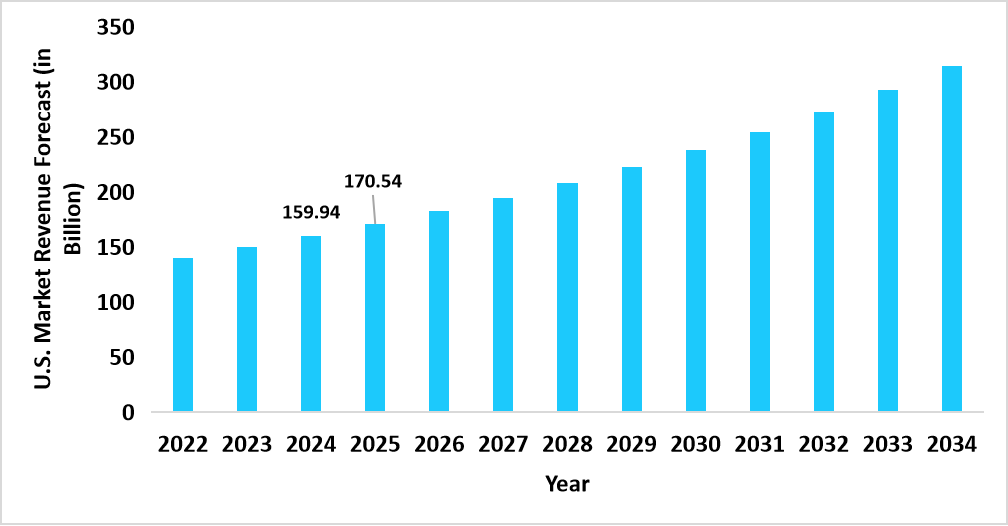
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 399.76 billion
- 2034 Projected Market Size: USD 736.06 billion
- CAGR (2025 to 2034): 7.06%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The global market encompasses a diverse range of biologically derived products designed to treat various chronic and life-threatening conditions by targeting specific molecular pathways. By product, the market is segmented into monoclonal antibodies, which dominate due to their precision in targeting disease specific antigens, insulin, widely used for diabetes management, fusion proteins, offering enhanced stability and therapeutic efficacy, erythropoietin, essential for anaemia and renal disorder treatment, interferons, utilized in antiviral and anticancer therapies, human growth hormone, supporting growth deficiency and metabolic regulation, and follicle stimulating hormone, primarily used in fertility treatments. By application, protein therapeutics play a crucial role in addressing metabolic disorders, immunologic disorders, hematological disorders, cancer, and genetic disorders, along with other conditions requiring advanced biologic interventions. The continuous advancement in protein engineering, improved formulation stability, and expanding applications in personalized medicine are collectively propelling the global growth and innovation of the market.
Latest Market Trends
Expansion of Biosimilars & Interchangeable Insulin Analogs
A trend in the protein therapeutics market is the shift from exclusive branded insulin analogues to biosimilar and interchangeable formulations, which improves access and lower cost for patients. For example, in July 2025, the U.S. FDA approved Kirsty (Insulin Aspart-xjhz), the first interchangeable rapid-acting insulin aspart biosimilar to Novolog, enabling substitution at the pharmacy level without prescriber intervention.
These developments highlighted the growing shift towards biosimilars and interchangeable insulin analogs, boosting affordability, competition, and patient access in diabetes care.
Expansion of Indications for Monoclonal Antibodies in Rare Diseases & Immune Disorders
A growing trend in the global market is the shift from using monoclonal antibodies (mAbs) mainly for common diseases (cancer, arthritis, etc.) to targeting rare diseases and immune disorders, which supports increasing drug indications and patient access. Recently, Amgen Inc.’s UPLIZNA (inebilizumab cdon) was approved by the U.S. FDA as the first treatment for Immunoglobulin G4-related disease (IgG4 RD), a rare, chronic immune-mediated condition, expanding its use beyond its prior indication for NMOSD.
These expansions of mAb indications for rare immune-mediated diseases are boosting market growth through unmet demands, which further broaden the therapeutic application.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 399.76 Billion |
| Estimated 2026 Value | USD 426.55 Billion |
| Projected 2034 Value | USD 736.06 Billion |
| CAGR (2026-2034) | 7.06% |
| Dominant Region | North America |
| Fastest Growing Region | Asia-Pacific |
| Key Market Players | Bayer AG, Bristol-Myers Squibb Company, DAIICHI SANKYO COMPANY, LIMITED, Abbott, Sanofi |
to learn more about this report Download Free Sample Report
Protein Therapeutics Market Drivers
Adoption of Personalized Protein Therapies in Oncology
The protein therapeutics market is witnessing strong growth in personalized therapies that are tailored to individual patient profiles. For instance, in April 2025, the U.S. FDA approved a novel monoclonal antibody, OncoMab 1, for the treatment of patients with HER2-positive metastatic breast cancer. This approval was based on the identification of specific genetic markers in tumor biopsies, allowing selection of patients most likely to benefit from the therapy.
Therefore, the personalized approach enhances treatment efficacy while minimizing adverse effects.
Market Restraint
High Cost of Development, Manufacturing Complexity, and Pricing Challenges
The protein therapeutics market faces challenges due to the high costs associated with development, complex manufacturing processes, and pricing pressures. These factors can limit the accessibility of therapies and impact market growth. As per the annual report of Amgen Inc., in 2024, the company highlighted the impact of existing and proposed state pricing laws on the complexity of drug pricing. The company noted that such legislation added complexity to the pricing of drugs and already affected industry pricing decisions.
Such regulatory actions affected reimbursement strategies and the financial viability of new protein therapeutics in the market.
Market Opportunities
Strategic Support for Monoclonal Antibodies
Growing strategic support for protein therapeutics, including monoclonal antibodies, is enhancing the global market growth by prioritizing clinically advanced and cost-efficient treatments. The table below depicts national spending in 2023/24 on protein-based therapies by the NHS in the UK.
Table: Nationally Prioritized Monoclonal Antibodies
|
Monoclonal Antibodies |
Indication |
Expenditure in year 2023/2024 |
|
Ustekinumab |
Crohn’s, psoriatic arthritis, ulcerative colitis |
USD 265.88 million
|
|
Ocrelizumab |
Multiple sclerosis |
USD 151.93 million
|
|
Ipilimumab |
Cancer |
USD 88.63 million
|
|
Vedolizumab |
Ulcerative colitis, Crohn’s |
USD 240.56 million
|
Source: Straits Research
Such a factor highlighted the strong market potential and reinforced the protein therapeutics market as a key pillar of modern healthcare.
Regional Analysis
The North America region dominated the market with a revenue share of 46.92 % in 2025, owing to factors such as the high adoption of advanced biologics, strong healthcare infrastructure, and government initiatives to enhance the development of advanced therapeutics, which further enhance the growth of the protein therapeutics market.
The market in the U.S. is widely driven by the FDA's accelerated approval pathway for breakthrough biologics. This policy expedites the review of drugs addressing unmet medical demands, hence reducing time to market. This regulatory support enhances protein therapeutics’ market size and fosters innovation in protein-based therapies.
Asia Pacific Market Insights
The Asia Pacific region is the fastest-growing region with a CAGR of 9.57% during the forecast timeframe. The growth is attributed to factors such as increasing healthcare expenditure, improving access to advanced biologic therapies, and rising prevalence of chronic and genetic disorders.
The protein therapeutics market in China is fuelled by policy changes easing foreign investment restrictions in the stem cell, gene therapy, and genetic diagnosis sectors within four Free Trade Zones: Beijing, Shanghai, Guangdong, and Hainan. These policies allow foreign enterprises to register, list, and produce products under Chinese regulations, accelerating advanced protein therapeutics development and market expansion.
Pie Chart: Regional Market Share, 2025
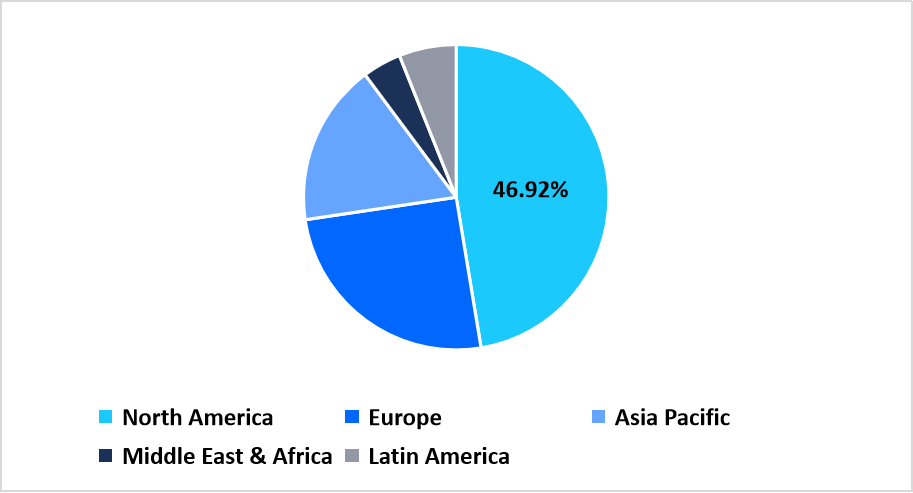
Source: Straits Research
Europe Market Insights
In Europe, the protein therapeutics market is driven by the government's commitment to enhancing the pharmaceutical sector. German Chancellor Olaf Scholz announced tax incentives for research and measures to reduce bureaucratic obstacles for clinical trials. This initiative aimed to foster innovation and expedite the development of protein-based therapies, thereby contributing to the expansion of the market.
The market in the UK is propelling due to the government’s £600 million investment in the Health Data Research Service. This initiative enhances access to NHS health data, accelerating clinical research and protein-based therapy development. By reducing trial setup times and supporting innovation, it further boosts the market size and growth of market.
Latin America Market Insights
A niche factor driving the growth of the protein therapeutics market in Latin America is the increasing adoption of biosimilars due to cost-sensitive healthcare systems. Governments and private healthcare providers in countries such as Brazil, Mexico, and Argentina are actively promoting biosimilars as a more affordable alternative to branded biologics, expanding patient access to protein-based therapies while driving market expansion in the region.
The government’s increasing support for local biopharmaceutical manufacturing drives the market growth. Argentina has implemented policies and incentives to promote domestic production of biologics and protein-based therapies, aiming to reduce dependency on imports and improve accessibility. This has encouraged both local and multinational companies to invest in protein therapeutics development and distribution within the country.
Middle East and Africa Market Insights
The growing focus on public-private partnerships to enhance biologics accessibility drives the market growth. Governments in countries like Saudi Arabia, the UAE, and South Africa are collaborating with international pharmaceutical companies to establish local manufacturing hubs and distribution networks for protein therapeutics, aiming to improve treatment availability for chronic and rare diseases in the region.
The adoption of precision medicine initiatives targeting genetic and immune-related disorders drives the market growth. Increasing investment in genomics and personalized healthcare is creating demand for protein-based therapies tailored to specific patient populations.
Product Insights
The monoclonal antibodies segment dominated the market in 2025 with a revenue share of 37.21%. The growth is attributed to the increasing adoption of monoclonal antibody therapies across oncology, immunologic, and hematologic disorders, driven by potent clinical efficacy.
The insulin segment is anticipated to register the fastest CAGR of 8.12% during the forecast period, owing to the increasing demand for recombinant and long-acting insulin formulations, and advancements in protein engineering that enhance insulin stability and patient compliance.

Source: Straits Research
Application Insights
The immunologic disorders segment is anticipated to grow at a CAGR of 8.93% during 2026-2034, owing to the increasing prevalence of autoimmune and inflammatory diseases, rising demand for targeted protein therapeutics such as monoclonal antibodies and fusion proteins, and the adoption of advanced biologics that improve patient outcomes and treatment adherence.
The metabolic disorders dominated the market in 2025, with a revenue share of 35.23% in 2025, owing to the increasing adoption of advanced insulin delivery technologies and personalized treatment plans, which have enhanced patient adherence and improved clinical outcomes.
Competitive Landscape
The global protein therapeutics market is highly fragmented with numerous multinational pharmaceutical companies, biotechnology firms, and contract development and manufacturing organizations (CDMOs) actively innovating in monoclonal antibodies, recombinant proteins, and enzyme therapies.
The industry participants are inclined towards forming strategic partnerships and investing in advanced biologics manufacturing technologies to strengthen their global footprint.
Alligator Bioscience AB.: An emerging market player
Alligator Bioscience AB is an emerging player in the market, focusing on advanced monoclonal antibodies for oncology and immune oncology indications.
- In April 2025, Alligator Bioscience AB announced the initiation of Phase II clinical trials for HLX22, an anti-HER2 monoclonal antibody developed by its subsidiary, Atlas Therapeutics, and licensed to Shanghai Henlius Biotech, Inc., targeting HER2-positive cancers.
List of Key and Emerging Players in Protein Therapeutics Market
- Bayer AG
- Bristol-Myers Squibb Company
- DAIICHI SANKYO COMPANY, LIMITED
- Abbott
- Sanofi
- Thermo Fisher Scientific, Inc.
- Alligator Bioscience AB
- AbbVie Inc.
- Takeda Pharmaceutical Company Limited.
- Amgen Inc.
- Hoffmann-La Roche Ltd
- Lilly USA
- Pfizer Inc.
- Novo Nordisk A/S
- Genmab A/S
- BioMarin
- CSL
- Regeneron Pharmaceuticals Inc.
- Vertex Pharmaceuticals Incorporated
- Kyowa Kirin Co., Ltd.
- Others
Strategic Initiatives
- September 2025: Glycomine announced a USD 115 million Series C financing to advance its lead drug candidate, GLM101, into a Phase 2b clinical trial for PMM2-CDG, a rare genetic disorder.
- June 2025: Shanghai Henlius Biotech, Inc. announced the approval of serplulimab for treating adults with extensive-stage small-cell lung cancer, which has not been previously treated in the body.
- June 2025: Amgen Inc. announced the launch of ProThera, a long-acting formulation of its monoclonal antibody TheraMab, designed for once-monthly subcutaneous administration.
- June 2025: Amgen reported that IMDELLTRA (tarlatamab-dlle) reduced the risk of death by 40% in patients with small cell lung cancer (SCLC) who had progressed on or after one line of platinum-based chemotherapy.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 399.76 Billion |
| Market Size in 2026 | USD 426.55 Billion |
| Market Size in 2034 | USD 736.06 Billion |
| CAGR | 7.06% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Applications |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Protein Therapeutics Market Segments
By Product
- Monoclonal Antibodies
- Insulin
- Fusion Protein
- Erythropoietin
- Interferon
- Human Growth Hormone
- Follicle Stimulating Hormone
By Applications
- Metabolic Disorders
- Immunologic Disorders
- Haematological Disorders
- Cancer
- Genetic Disorders
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































