Pyrogen Testing Market Size, Share & Trends Analysis Report By Type (Rabbit Pyrogen Test, Limulus Amebocyte Lysate Test , Monocyte Activation Test ), By Product (Consumables, Instruments, Services), By End-User (Pharmaceutical and Biotechnology Companies, Medical devices companies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2025-2033
Pyrogen Testing Market Size
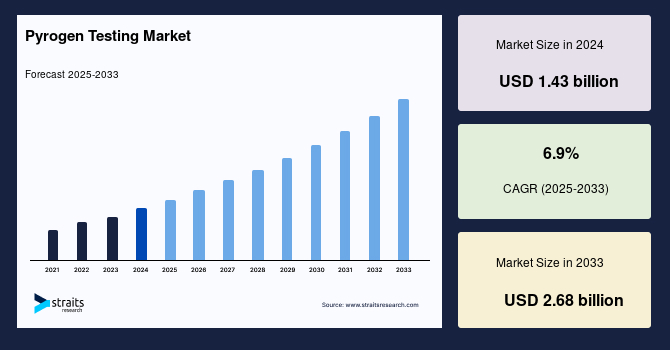
The global pyrogen testing market size was valued at USD 1.43 billion in 2024 and is projected to grow from USD 1.57 billion in 2025 to reach USD 2.68 billion by 2033, exhibiting a CAGR of 6.9% during the forecast period (2025-2033).
Pyrogen Testing is a critical process for detecting and measuring pyrogens substances that can cause fever when introduced into the body. These pyrogens, often bacterial endotoxins, are commonly found in medical products like drugs, vaccines, and medical devices. Pyrogen testing ensures that these products are free from fever-inducing contaminants, thus safeguarding patient health. Common methods of pyrogen testing include the LAL (Limulus Amebocyte Lysate) test and the Rabbit Pyrogen Test (RPT), both of which focus on detecting endotoxins and other harmful compounds.
The market is expanding rapidly, driven by the growing production of biologics, injectables, and medical devices that require precise endotoxin detection. Regulatory mandates from agencies like the FDA, EMA, and USP are pushing for the adoption of advanced pyrogen testing methods. This includes the transition from traditional animal-based tests like the Rabbit Pyrogen Test (RPT) to in vitro alternatives, such as the Monocyte Activation Test (MAT) and recombinant Factor C (rFC) assays.
The growing prevalence of infectious diseases, coupled with expanding biopharmaceutical manufacturing, is significantly boosting the demand for efficient pyrogen detection solutions. Technological advancements, including automated endotoxin detection systems and AI-driven data analysis, are improving testing accuracy and operational efficiency. Moreover, emerging opportunities lie in the development of cost-effective in vitro testing kits and the expansion of contract testing services to support small and mid-sized pharmaceutical companies.
Latest Market Trends
Shift toward in Vitro Pyrogen Testing
The pyrogen testing market is increasingly transitioning from traditional animal-based methods like the Rabbit Pyrogen Test (RPT) to more ethical and efficient in vitro techniques, such as the Monocyte Activation Test (MAT). This shift is driven by ethical concerns and increasing regulatory support from bodies like the FDA and EMA, which promote alternative methods offering more reliable, reproducible results.
- For example, in June 2024, FUJIFILM Wako Pure Chemicals Corporation launched the LumiMAT Pyrogen Detection Kit a next-generation MAT for in vitro pyrogen testing and PYROSTAR Neo+, a recombinant protein reagent for detecting bacterial endotoxins. These innovations present effective alternatives to traditional pyrogen tests, making it easier to comply with ethical guidelines and regulatory standards.
This growing shift toward advanced in vitro methods is expected to enhance testing accuracy, improve regulatory compliance, and boost the market for pyrogen testing globally.
Advancement in Detection Techniques
The global market is also benefiting from continuous advancements in detection techniques, which increase accuracy, sensitivity, and efficiency. Emerging technologies, such as recombinant Factor C (rFC)-based assays, automated endotoxin detection systems, and AI-driven data analysis, are revolutionizing pyrogen testing by streamlining processes and improving outcomes.
- For instance, a February 2024 study published in the National Library of Medicine introduced a novel transgenic cell line-based method for pyrogen detection, which offers a more ethical, reliable, and scalable alternative to traditional methods. The study highlighted its high sensitivity and reproducibility, positioning it as a promising solution aligned with current regulatory standards.
These technological advancements are encouraging the adoption of sustainable, accurate, and scalable detection solutions, ensuring compliance with evolving regulations and enhancing overall product safety.
Market Summary
| Market Metric | Details & Data (2024-2033) |
|---|---|
| 2024 Market Valuation | USD 1.43 Billion |
| Estimated 2025 Value | USD 1.57 Billion |
| Projected 2033 Value | USD 2.68 Billion |
| CAGR (2025-2033) | 6.9% |
| Dominant Region | North America |
| Fastest Growing Region | as |
| Key Market Players | Charles River Laboratories, Inc, Lonza, Merck KGaA, Thermo Fisher Scientific, Inc., Associates of Cape Cod, Inc. |
to learn more about this report Download Free Sample Report
Pyrogen Testing Market Driving Factors
Growth of the Medical Device Industry
The expanding medical device industry is driving increased demand for pyrogen testing as the production of implantable devices and surgical instruments grows. Regulatory bodies mandate stringent pyrogen and endotoxin testing to ensure compliance with safety standards and protect patient health.
- For example, in January 2025, BD announced that it would increase investments in its U.S. manufacturing network to boost capacity for critical medical devices such as syringes, needles, and IV catheters. This move addresses growing healthcare system demands while ensuring that medical devices meet pyrogen safety standards.
Such investments and the continuous expansion of the medical device manufacturing sector are expected to drive the demand for reliable pyrogen testing solutions globally.
Rising Incidence of Chronic Diseases
The increasing prevalence of chronic diseases, such as cancer, diabetes, and cardiovascular conditions, is significantly raising the demand for biologics, vaccines, and injectable therapies. These treatments require rigorous pyrogen and endotoxin testing to ensure safety and meet regulatory requirements.
- For example, in February 2024, the Centers for Disease Control and Prevention(CDC) reported that 129 million people in the U.S. have at least one chronic disease, fueling demand for biologics and injectable therapies. This growing disease burden is expected to accelerate the need for advanced pyrogen testing, ensuring that critical therapies are both safe and effective for consumers.
As a result, the market for pyrogen testing will continue to grow in response to the increasing need for safe, high-quality treatments.
Market Restraining Factors
Complex Regulatory Approval Processes
One of the key restraining factors in the global pyrogen testing market is the complex and stringent regulatory approval processes. Manufacturers must comply with rigorous guidelines set by agencies such as the FDA, EMA, and USP, which require extensive validation, documentation, and quality control to ensure the safety and efficacy of testing methods. These processes can result in significant delays, increased operational costs, and hinder the market entry of new products. Moreover, the continuous need to adapt to evolving regulatory standards and implement frequent updates to testing protocols adds complexity, further limiting the swift adoption of innovative pyrogen testing solutions.
Market Opportunity
Expansion of Biopharmaceutical Manufacturing
The rapid expansion of biopharmaceutical manufacturing represents a significant opportunity for the market. The increasing production of biologics, such as monoclonal antibodies, gene therapies, and biosimilars, requires stringent quality control, including reliable pyrogen detection methods.
- For instance, in July 2022, Merck opened its first Microbiology Application and Training (MAT) Lab in Bengaluru, India, to bolster microbial quality control capabilities in the Indian life science sector. This €200,000 investment supports essential services like sterility testing, rapid bioburden testing, pyrogen testing, and advanced membrane filtration, addressing the growing needs of the biopharmaceutical and pharmaceutical industries.
The ongoing expansion of biopharmaceutical manufacturing facilities, particularly in emerging markets, is expected to drive further demand for advanced pyrogen testing solutions, ensuring the safety, compliance, and quality of biopharma products.
Regional Insights
North America Pyrogen Testing Market: Dominant Region with 40.9% Market Share
North America holds a leading position in the global pyrogen testing market due to stringent regulatory frameworks enforced by the FDA and USP, along with high adoption of Limulus Amebocyte Lysate (LAL) assays. The region benefits from extensive pharmaceutical R&D, advanced biopharmaceutical production, and the presence of leading biotechnology companies. Moreover, the increasing use of in vitro testing methods, such as the Monocyte Activation Test (MAT), is gaining regulatory approval, further boosting market growth.
- The U.S. pyrogen testing market is driven by the expansion of new facilities and an increase in investments in the country. In July 2024, Nipro Medical Corporation announced its expansion in the U.S. with the establishment of its first manufacturing facility in Greenville, U.S. This strategic move involves an investment of approximately USD 398 million over the next five years and is expected to create 232 new jobs, boosting local employment and enhancing the company’s manufacturing capabilities and product supply across the U.S.
Asia-Pacific Pyrogen Testing Market: Fastest Growing Region with the Highest Market Cagr
Asia-Pacific region is expected to register the fastest CAGR due to rising pharmaceutical manufacturing, increased government investments in healthcare, and the expansion of biotechnology research. Emerging economies such as China and India are witnessing a surge in biologics production, necessitating stringent pyrogen testing protocols. The region also benefits from growing regulatory awareness and increased adoption of in vitro alternatives to animal-based testing.
- India’s pyrogen testing market is driven by government initiatives and the expansion of biomanufacturing facilities. In March 2023, Wipro GE Healthcare launched its new Medical Device Manufacturing (MDM) facility in Bengaluru, India, under the Indian government’s Production Linked Incentive (PLI) Scheme. This facility boosts local medical device manufacturing and supports the Atmanirbhar Bharat initiative, strengthening India’s self-reliance in healthcare technology.
Europe Pyrogen Testing Market: A Significant Market Driven by Strong Research and Development
Europe represents a key market for pyrogen testing due to the region’s emphasis on compliance with European Pharmacopoeia (EP) guidelines and a growing focus on replacing animal-based tests with in vitro alternatives. Increased funding for biotechnology research and growing biopharmaceutical production further fuel the demand for pyrogen testing. Moreover, the region is witnessing a gradual transition to the Monocyte Activation Test (MAT) due to ethical concerns over animal testing.
- Germany’s pyrogen testing market is driven by mergers and acquisitions among key players in the country. For instance, in January 2025, Cormica, a life sciences testing and consulting company, acquired Zwisler Laboratorium, a premier laboratory services provider in Reichenau, Germany. This acquisition marks Cormica's entry into the German market, strengthening its presence in high-growth European markets and supporting future expansion.
- France’s market for pyrogen testing is driven by an increase in investments and the expansion of biological facilities to increase production, which drives the pyrogen testing industry. In September 2024, Sanofi inaugurated Modulus, a new production unit in Neuville-sur-Saône, France, supported by a €500 million investment. Modulus utilizes a world-first modular concept that can adapt to produce up to four vaccines simultaneously.
Segmentation Analysis
By Type
The Limulus Amebocyte Lysate (LAL) assay leads the global market due to its high sensitivity, reliability, and widespread regulatory acceptance for detecting bacterial endotoxins in injectable products and medical devices. This method is favored across the pharmaceutical and biotechnology industries for its rapid results and ease of use. Recent technological advancements, including recombinant Factor C (rFC) alternatives, are further promoting its adoption by reducing reliance on horseshoe crab blood, thus ensuring more sustainable and efficient pyrogen testing processes.
By Product
Consumables dominate the market, driven by the continuous demand for Limulus Amebocyte Lysate reagents, endotoxin detection kits, and culture media used in testing procedures. These consumables are critical for routine quality control in pharmaceutical and medical device manufacturing. The increasing adoption of advanced technologies like MAT and recombinant methods has spurred the demand for specialized consumables. Moreover, the rise of biopharmaceutical manufacturing and strict regulatory standards further fuels the need for consistent, high-quality consumables to maintain product safety.
By End-User
Pharmaceutical and biotechnology companies are the largest end-users in the market due to the rising production of biologics, biosimilars, and parenteral drugs, all of which require stringent pyrogen testing. These companies rely on pyrogen testing to ensure product safety and meet regulatory compliance. The surge in clinical trials, drug development, and the growing adoption of personalized and gene therapies are further intensifying the need for reliable endotoxin detection and pyrogen control, fueling the demand for pyrogen testing services in these sectors.
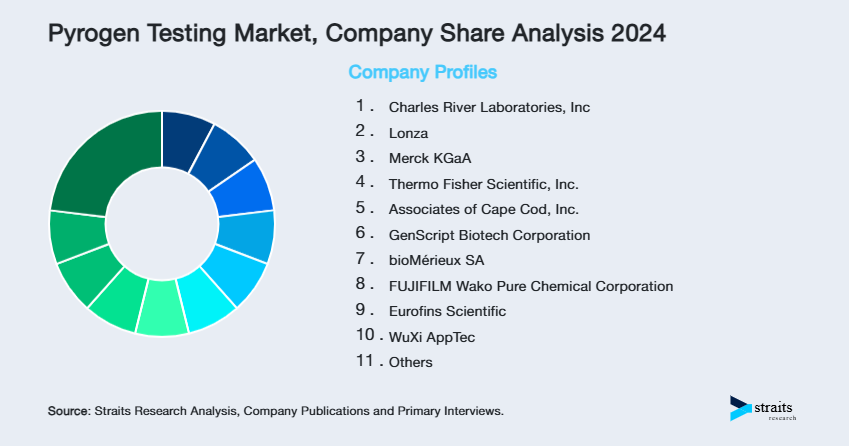
Company Market Share
The market is moderately fragmented, with key players prioritizing innovation, regulatory compliance, and strategic partnerships to enhance their market presence. Leading companies are focusing on expanding their portfolios by introducing recombinant and MAT-based testing solutions, broadening their market reach. Moreover, significant investments in automation, animal-free testing methods, and emerging technologies are positioning these players to meet the growing demand for efficient and ethical pyrogen testing.
Lonza: An Emerging Player in the Global Pyrogen Testing Market
Lonza is a leading life sciences company providing contract manufacturing and development services. In the market, Lonza offers advanced endotoxin and pyrogen detection solutions, such as LAL and TAL assays and the Nebula Absorbance Reader, ensuring accurate quality control in pharmaceutical manufacturing.
Recent Developments at Lonza:
- In October 2023, Lonza launched two new rapid Monocyte Activation Test (MAT) systems, PyroCell MAT Rapid System and PyroCell MAT Human Serum (HS) Rapid System, which reduced testing time from two days to two hours. These systems, featuring the PeliKine Human IL-6 Rapid ELISA Kit, offer faster, more efficient MAT testing for product safety while reducing reliance on animal-based methods.
List of Key and Emerging Players in Pyrogen Testing Market
- Charles River Laboratories, Inc
- Lonza
- Merck KGaA
- Thermo Fisher Scientific, Inc.
- Associates of Cape Cod, Inc.
- GenScript Biotech Corporation
- bioMérieux SA
- FUJIFILM Wako Pure Chemical Corporation
- Eurofins Scientific
- WuXi AppTec
- Hycult Biotech
- Cormica Ltd
- BD
- Nelson Laboratories, LLC
- Indoor Biotechnologies, Inc
to learn more about this report Download Market Share
Recent Developments
- June 2024 – Freudenberg Medical, a global contract development and manufacturing organization for medical devices and biopharma, invested over USD 50 million in a new production facility in Aachen, Germany. This investment enhances Freudenberg Medical’s capabilities, especially for drug-device combination products like drug coatings for balloon catheters and stents, further elevating the demand for stringent pyrogen testing to ensure safety and compliance.
- January 2024 –Charles River Laboratories launched the Endosafe Trillium rCR cartridge, combining its Endosafe cartridge technology with a recombinant cascade reagent (rCR) to offer an animal-free bacterial endotoxin testing (BET) solution. This innovation enhances testing efficiency, reduces reliance on animal-derived reagents, and accelerates manufacturing timelines, positioning the company as a leader.
Analyst Opinion
As per our analyst, the global pyrogen testing market is set to experience substantial growth, driven by the rising production of pharmaceuticals and biologics coupled with stringent regulatory requirements. The increasing adoption of in vitro testing methods, such as the Monocyte Activation Test (MAT), is reshaping the landscape, offering more reliable and ethical alternatives to traditional methods.
However, despite these positive trends, the market faces challenges, including the complexity of regulatory approval processes and the high costs associated with advanced testing methods. Additionally, transitioning from traditional testing methods, such as Limulus Amebocyte Lysate (LAL), to recombinant Factor C (rFC)-based assays presents technical hurdles and requires substantial investment in new technologies.
Nonetheless, the ongoing advancements in recombinant technologies and automation, along with expanding biopharmaceutical infrastructure, particularly in emerging markets like Asia-Pacific, present significant growth opportunities. Increased government focus on quality control in drug manufacturing is expected to further drive demand for pyrogen testing, positioning these regions as key players in the market’s future expansion.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2024 | USD 1.43 Billion |
| Market Size in 2025 | USD 1.57 Billion |
| Market Size in 2033 | USD 2.68 Billion |
| CAGR | 6.9% (2025-2033) |
| Base Year for Estimation | 2024 |
| Historical Data | 2021-2023 |
| Forecast Period | 2025-2033 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Product, By End-User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Pyrogen Testing Market Segments
By Type
- Rabbit Pyrogen Test
- Limulus Amebocyte Lysate Test
- Monocyte Activation Test
By Product
- Consumables
- Instruments
- Services
By End-User
- Pharmaceutical and Biotechnology Companies
- Medical devices companies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Dhanashri Bhapakar
Senior Research Associate
Dhanashri Bhapakar is a Senior Research Associate with 3+ years of experience in the Biotechnology sector. She focuses on tracking innovation trends, R&D breakthroughs, and market opportunities within biopharmaceuticals and life sciences. Dhanashri’s deep industry knowledge enables her to provide precise, data-backed insights that help companies innovate and compete effectively in global biotech markets.










































