Respiratory Syncytial Virus Diagnostics Market Size, Share & Trends Analysis Report By Product (Kits & Assays, Instruments, Others), By Method (Direct Fluorescent Antibody (DFA) Method, Flow Cytometry, Molecular Diagnostics, Diagnostic Imaging, Chromatographic Immunoassay, Rapid Antigen Diagnostic Test (RADTs), Others), By End User (Hospitals & Clinics, Diagnostic & Clinical Laboratories, Homecare) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Respiratory Syncytial Virus Diagnostics Market Overview
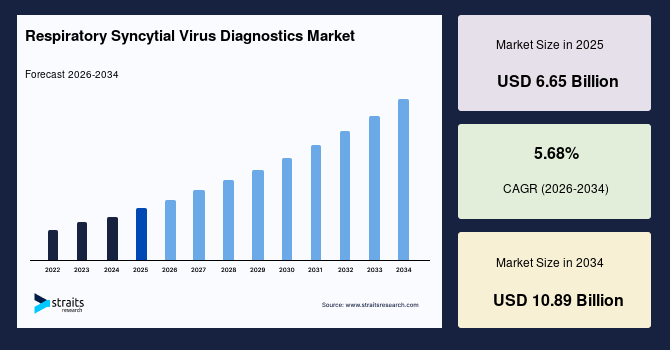
The global respiratory syncytial virus diagnostics market size is estimated at USD 6.65 billion in 2025 and is projected to reach USD 10.89 billion by 2034, growing at a CAGR of 5.68% during the forecast period. The remarkable market growth is attributed to the integration of RSV diagnostics into antimicrobial stewardship programs, which enable rapid viral detection, reducing unnecessary antibiotic use, and enhancing patient management efficiency.
Key Market Trends & Insights
- North America held a dominant share of the global market, accounting for 41.19% share in 2025.
- The Asia Pacific region is estimated to grow at the fastest pace, with a CAGR of 7.03% during the forecast period.
- Based on product, the kits & assays segment dominated the market, accounting for 46.56% of the revenue share in 2025.
- By method, the chromatographic immunoassay segment dominated the market with a revenue share of 26.94% in 2025.
- By end-user, the hospitals & clinics segment dominated the market in 2025, with a revenue share of 43.07%.
- The U.S. dominates the market, valued at USD 2.34 billion in 2024 and reaching USD 2.47 billion in 2025.
Table: U.S. Respiratory Syncytial Virus Diagnostics Market Size (USD Million)
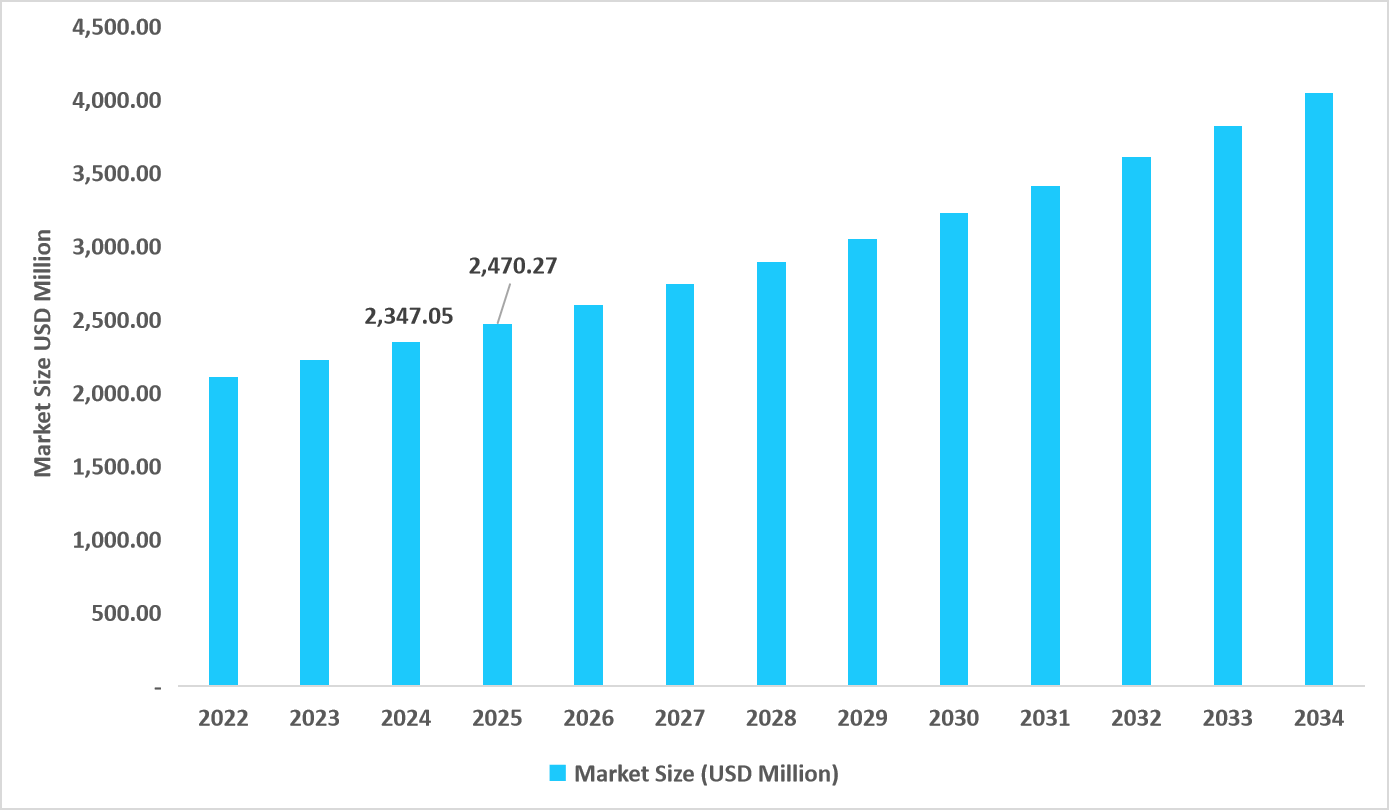
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 6.65 billion
- 2034 Projected Market Size: USD 10.89 billion
- CAGR (2026-2034): 5.68%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The global respiratory syncytial virus diagnostics market encompasses a range of products, including kits & assays, instruments, and others, designed to detect RSV infections across diverse clinical settings. The market leverages multiple diagnostic methods such as molecular diagnostics, chromatographic immunoassays, flow cytometry, DFA, RADTs, and imaging techniques. Key end users include hospitals & clinics, diagnostic and clinical laboratories, and home care, reflecting an integration of advanced testing technologies for timely and accurate RSV detection.
Latest Market Trends
Rapid Adoption of Multiplex Molecular Panels
A major trend in the RSV diagnostics market is the rapid adoption of multiplex molecular panels that simultaneously detect RSV along with influenza, SARS-CoV-2, and other respiratory pathogens. These advanced panels offer faster and comprehensive results than traditional single-pathogen tests, enabling clinicians to make timely treatment decisions during overlapping respiratory seasons. The growing clinical preference for integrated testing drives higher utilization across hospitals and urgent care centers, strengthens demand for modern molecular platforms, and supports market expansion.
Shift Towards Point-of-care Molecular Testing
The expanding shift toward point-of-care molecular testing, which offers rapid, highly sensitive detection directly in clinical and community settings. This shift is driven by the demand for faster decision-making during peak RSV seasons and the growing availability of portable molecular platforms. For instance, companies have introduced compact PCR devices capable of delivering RSV results within 20–30 minutes, enabling urgent care centers and outpatient clinics to manage high-risk patients more efficiently and reduce hospital burden.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 6.65 Billion |
| Estimated 2026 Value | USD 7.01 Billion |
| Projected 2034 Value | USD 10.89 Billion |
| CAGR (2026-2034) | 5.68% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | BD, Abbott, BIOMÉRIEUX, Bio-Rad Laboratories, Inc., Coris BioConcept |
to learn more about this report Download Free Sample Report
Market Drivers
Rising Integration of Routine RSV Screening in Respiratory Care
A key driver in the respiratory syncytial virus diagnostics market is the accelerating shift toward routine RSV screening as part of integrated respiratory disease management, fueled by the growing availability of RSV vaccines and monoclonal antibodies. As prevention strategies expand, healthcare providers increasingly rely on diagnostic testing to guide eligibility, monitor breakthrough infections, and support population-level surveillance. For example, following the introduction of adult RSV vaccines in the U.S., hospitals reported higher demand for diagnostic confirmation to support clinical decisions and public health reporting, directly boosting the requirement for RSV testing solutions.
Market Restraints
The High Cost of Molecular Platforms Limits Broader Market Adoption
A major restraint in the RSV diagnostics market is the high cost of advanced molecular testing platforms, which limits broad adoption across lower-resource healthcare settings despite rising diagnostic demand. Many hospitals and clinics face budget constraints that restrict investment in high-throughput PCR systems and multiplex respiratory panels. For instance, during the 2023-2024 RSV season, several community clinics in Latin America reported reliance on older antigen tests due to the prohibitively high cost of molecular instruments, underscoring how affordability challenges continue to hinder market expansion.
Market Opportunity
Growing Adoption of At-home and Decentralized RSV Testing
A key opportunity in the RSV diagnostics market is the rapid expansion of at-home and decentralized testing, driven by rising demand for convenient, accessible respiratory diagnostics. Consumers and outpatient centers increasingly prefer self-administered or near-patient testing options that reduce clinic visits during peak RSV seasons. For instance, several manufacturers introduced home-based multiplex respiratory test kits in 2024, enabling users to detect RSV alongside influenza and COVID-19. This shift opens a large, untapped retail and telehealth-driven market segment for RSV diagnostics.
Regional Analysis
North America dominated the market in 2025, accounting for 41.19% market share. This growth is driven by advanced government-led RSV surveillance programs in North America, which encourage routine testing in hospitals and community clinics. Initiatives such as CDC-supported respiratory monitoring networks promote early detection, generate high testing volumes, and create strong demand for innovative RSV diagnostic solutions across the region.
Canada's respiratory syncytial virus diagnostics market growth is attributed to the expansion of telehealth-integrated RSV testing, where remote clinics and virtual care providers use connected diagnostic platforms to monitor and report RSV cases. Such digital integration enhances accessibility in rural and northern regions, increasing testing adoption and supporting timely public health interventions.
Asia Pacific Market Insights
Asia Pacific is emerging as the fastest-growing region with a CAGR of 7.03% from 2026-2034, owing to the rising prevalence of RSV among aging populations in countries like Japan and South Korea, which is prompting healthcare providers to invest in advanced molecular and multiplex diagnostic platforms for timely detection and management.
China's respiratory syncytial virus diagnostics market expanded steadily, and this growth is attributed to rapid expansion of private pediatric and maternity hospitals implementing advanced RSV screening protocols, catering to a growing middle-class population seeking premium healthcare services. This focus on early respiratory disease detection in private care facilities is uniquely accelerating market demand in the country.
Regional Market share (%) in 2025
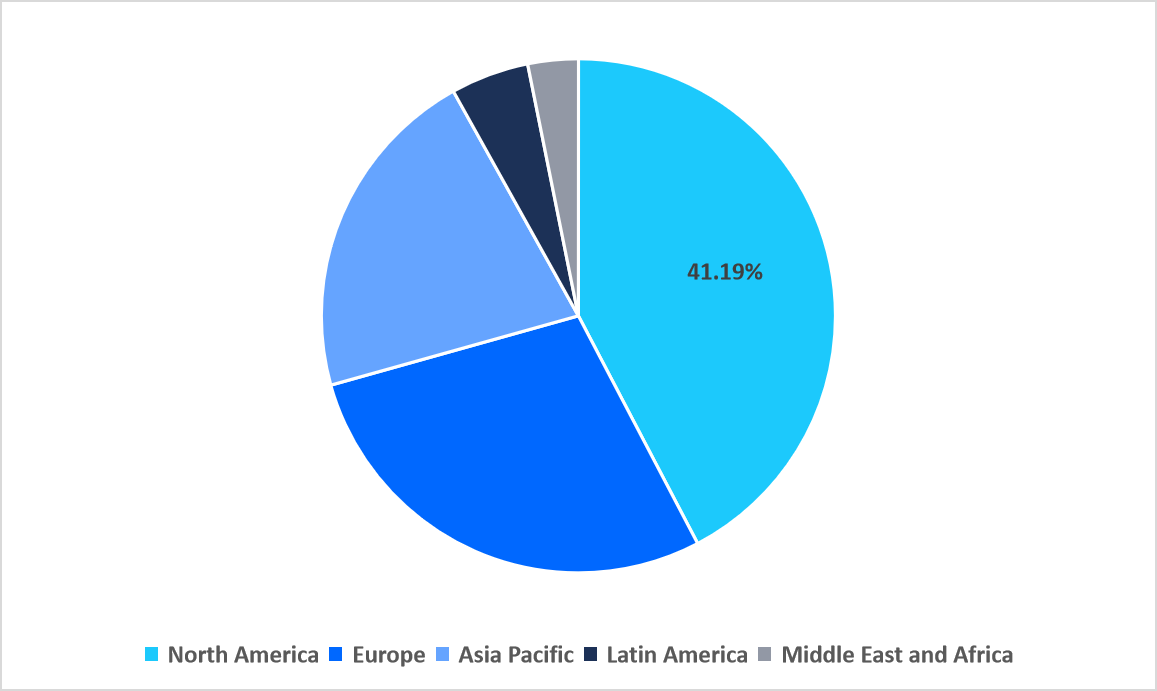
Source: Straits Research
Europe Market Insights
Europe’s market growth is supported by government-supported funding for pediatric RSV screening programs in countries like Germany and France. These initiatives promote early detection in newborn infants, driving adoption of advanced diagnostic kits and molecular assays across hospitals and specialized pediatric care centers.
German market is witnessing growth due to the integration of RSV testing into national influenza surveillance networks, enabling simultaneous monitoring of multiple respiratory pathogens. This approach allows public health authorities to track seasonal outbreaks efficiently, prompting hospitals and laboratories to adopt advanced multiplex diagnostic platforms, thereby boosting market demand.
Latin America Market Insights
Latin American market is witnessing steady growth, due to increasing collaborations between local laboratories and international diagnostic companies to improve access to quality RSV testing. These partnerships facilitate technology transfer, training, and distribution of advanced diagnostic kits, enhancing regional testing capacity and market expansion.
Argentina’s RSV diagnostics market growth is driven by the expansion of the National Laboratory Network to include real-time PCR based RSV testing across 46 regional laboratories. This government-led initiative enhances disease surveillance, increases testing volumes, and encourages adoption of high-sensitivity molecular diagnostic platforms, uniquely boosting market demand nationwide.
Middle East and Africa Market Insights
The Middle East and Africa respiratory syncytial virus diagnostics market is expanding due to the high prevalence of RSV infections among hospitalized children, particularly in sub-Saharan Africa, where rates reach up to 23%. This elevated disease burden is prompting healthcare systems to expand testing capacity and adopt advanced diagnostic solutions, uniquely boosting regional market demand.
The RSV diagnostics market in Saudi Arabia and Africa is driven by the high economic burden associated with delayed RSV diagnosis, which increases healthcare costs for severe respiratory infections. This has prompted healthcare providers and policymakers to expand early and accurate RSV testing, boosting demand for diagnostic solutions across the region.
Product Insights
The kits & assays segment dominated the market with a revenue share of 46.56% in 2025. This growth is attributed to increasing preference for ready-to-use RSV testing, as kits and assays enable faster deployment during peak respiratory seasons. Their standardized components streamline laboratory workflows, reduce preparation time, and allow facilities to scale testing volumes efficiently without additional equipment investments.
The instruments segment is projected to register the fastest CAGR growth of 6.31% during the forecast period, owing to the rising adoption of automated high-throughput analyzers that enable continuous, large-volume RSV testing in clinical laboratories. These advanced instruments enhance processing speed, reduce manual intervention, and support laboratories in managing growing seasonal testing demands with improved accuracy and operational efficiency.
By Product Type Market Share (%), 2025
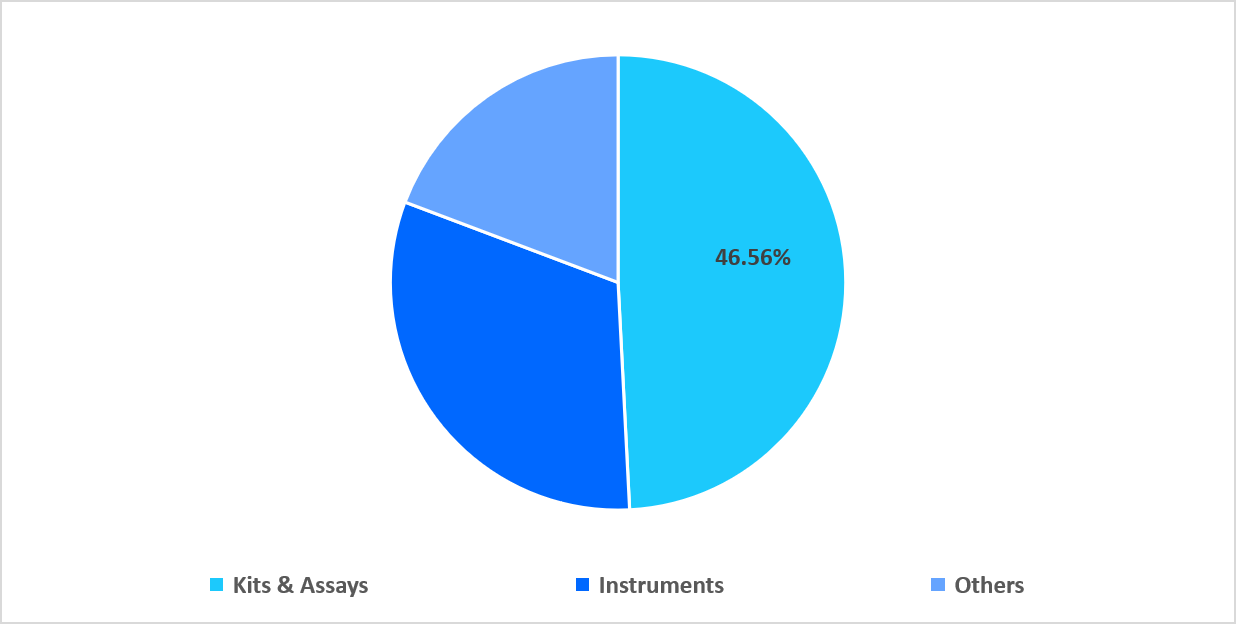
Source: Straits Research
Method Insights
The chromatographic immunoassay segment dominated the market in 2025 with a revenue share of 26.94%. This dominance is attributed to the high operational flexibility of chromatographic immunoassays, as they require no specialized instruments and maintain strong performance even in mobile healthcare settings. Their long shelf life and reliable stability make them a preferred choice for widespread RSV screening programs.
The molecular diagnostics segment is estimated to grow at the fastest CAGR of 6.28% during the forecast period. This growth is supported by a rising shift toward high-sensitivity RSV detection, as molecular diagnostics identify low viral loads. Their superior analytical accuracy makes them essential for confirming infections in high-risk patients, driving sustained adoption across advanced clinical laboratories.
End User Insights
The hospitals & clinics segment dominated the market in 2025 with a revenue share of 43.07%, as these facilities benefit from integrated laboratory infrastructure that supports continuous RSV testing during seasonal outbreaks, enabling rapid diagnosis, patient monitoring, and timely infection control measures.
The diagnostic & clinical laboratories segment is expected to register the fastest CAGR growth during the forecast period. This growth is driven by increasing centralization of high-volume RSV testing in diagnostic and clinical laboratories, where specialized equipment and skilled personnel allow for efficient processing of large sample volumes, improving turnaround times and supporting widespread surveillance during peak respiratory seasons.
Competitive Landscape
The global respiratory syncytial virus diagnostics market is moderately consolidated, with major players such as Abbott, F. Hoffmann-La Roche Ltd., Danaher, bioMérieux, QuidelOrtho Corporation, and others commanding a notable share through extensive product portfolios and strong laboratory networks. These companies focus on advancing molecular and rapid point-of-care technologies, securing regulatory approvals, and expanding global distribution. Meanwhile, emerging participants enhance competition by introducing cost-effective rapid tests and forming strategic collaborations to strengthen their market presence.
Coris BioConcept: An Emerging Market Player
Coris BioConcept is an emerging participant in the global market, gaining attention for its focus on affordable and rapid immunochromatographic testing solutions. As a smaller, innovative company, it is expanding its presence through targeted RSV diagnostic offerings designed for decentralized and resource-limited settings. With growing adoption of its rapid test kits across select international markets, Coris BioConcept is steadily strengthening its position as a rising competitor in RSV diagnostics.
List of Key and Emerging Players in Respiratory Syncytial Virus Diagnostics Market
- BD
- Abbott
- BIOMÉRIEUX
- Bio-Rad Laboratories, Inc.
- Coris BioConcept
- Creative Diagnostics
- Danaher
- DiaSorin S.p.A.
- Hoffmann-La Roche Ltd.
- Hologic, Inc.
- LumiraDx Ltd.
- Merck KGaA
- Meridian Bioscience, Inc.
- Novartis AG
- Quest Diagnostics Incorporated
- QuidelOrtho Corporation
- Siemens Healthcare S.A.
- Thermo Fisher Scientific Inc.
- Others
Strategic Initiatives
- May 2025: SEKISUI Diagnostics, a global medical diagnostics manufacturer, launched a new rapid diagnostic testing tool to detect Respiratory Syncytial Virus (RSV).
- October 2024: QIAGEN received the U.S. FDA approval for the QIAstat-Dx Respiratory Panel Mini test, which is designed to aid decision-making in diagnosing upper respiratory infections in outpatient settings.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 6.65 Billion |
| Market Size in 2026 | USD 7.01 Billion |
| Market Size in 2034 | USD 10.89 Billion |
| CAGR | 5.68% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Product, By Method, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Respiratory Syncytial Virus Diagnostics Market Segments
By Product
- Kits & Assays
- Instruments
- Others
By Method
- Direct Fluorescent Antibody (DFA) Method
- Flow Cytometry
- Molecular Diagnostics
- Diagnostic Imaging
- Chromatographic Immunoassay
- Rapid Antigen Diagnostic Test (RADTs)
- Others
By End User
- Hospitals & Clinics
- Diagnostic & Clinical Laboratories
- Homecare
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































