Imaging Biomarkers Market Size, Share & Trends Analysis Report By Type (Anatomical biomarkers, Functional biomarkers, Molecular/Nuclear Biomarkers, Quantitative Imaging Biomarkers, Others), By Technology (Positron Emission Tomography (PET), Ultrasound, Magnetic Resonance Imaging, Computed Tomography (CT), Single-Photon Emission Computerized Tomography (SPECT), Others), By Application (Diagnostics, Drug Discovery & Development, Personalized Medicine, Disease Risk Assessment, Others), By End User (Hospitals, Diagnostic/imaging centers, Pharmaceutical and Biotech Companies, Others) and By Region (North America, Europe, APAC, Middle East and Africa, LATAM) Forecasts, 2026-2034
Imaging Biomarkers Market Overview
The global imaging biomarkers market size is valued at USD 14.15 billion in 2025 and is estimated to reach USD 40.13 billion by 2034, growing at a CAGR of 12.32% during the forecast period. Strong growth of the market is due to the increasing integration of imaging biomarkers into companion diagnostics, which allows clinicians to correlate imaging findings with specific molecular targets and therapy responses.
Key Market Trends & Insights
- North America dominated the market with a revenue share of 36.43% in 2025.
- Asia Pacific is expected to grow at the fastest CAGR of 14.46% during the forecast period.
- Based on type, the molecular/nuclear biomarkers segment held the largest market share of 36.63% in 2025.
- By technology, the computed tomography (CT) segment dominated the market with a revenue share of 32.34%.
- Based on application, the diagnostics segment dominated the market with a revenue share of 37.38% in 2025.
- Based on end user, the hospitals segment dominated the market in 2025 with a revenue share of 41.27%.
- The U.S. dominates the imaging biomarkers market, valued at USD 4.22 billion in 2024 and reaching USD 4.73 billion in 2025.
Table: U.S. Imaging Biomarkers Market Size (USD Billion)
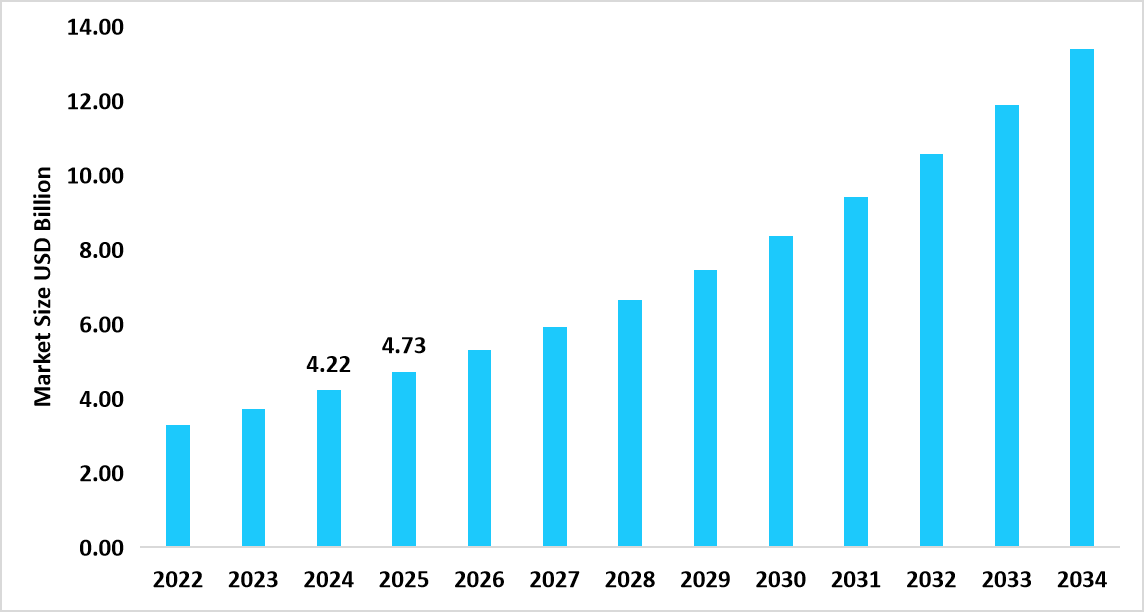
Source: Straits Research
Market Size & Forecast
- 2025 Market Size: USD 14.15 billion
- 2034 Projected Market Size: USD 40.13 billion
- CAGR (2026 2034): 12.32%
- Dominating Region: North America
- Fastest Growing Region: Asia Pacific
The imaging biomarkers market encompasses the development, validation, and application of quantifiable biological indicators derived from medical images to assess physiological processes, disease progression, and therapeutic response. Utilizing advanced imaging modalities such as Positron Emission Tomography (PET), Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Ultrasound, and Single-Photon Emission Computed Tomography (SPECT), imaging biomarkers enable objective evaluation of structural, functional, and molecular changes within the body.
The market spans diverse applications, including diagnostics, drug discovery and development, personalized medicine, and disease risk assessment, supporting early detection, precise treatment planning, and improved clinical outcomes. Major end users include hospitals, diagnostic and imaging centers, and pharmaceutical and biotechnology companies, which employ imaging biomarkers for clinical diagnosis, therapy monitoring, and drug validation in clinical trials. By integrating imaging science with data analytics and molecular research, the imaging biomarkers market is driving the evolution of evidence-based and precision-driven healthcare globally.
Latest Market Trends
Shift from qualitative imaging interpretation to quantitative biomarker-driven assessment
The imaging biomarkers market is witnessing a transformation from subjective, visual interpretation of medical images to quantitative, data-driven evaluation. Earlier, imaging primarily relied on radiologists' expertise for visual assessment, which often varied across observers and lacked reproducibility. The shift toward quantitative imaging biomarkers integrates image-derived metrics such as texture, perfusion, and metabolism into measurable parameters. This transition enables standardized disease characterization, enhances diagnostic accuracy, and supports the development of imaging-based endpoints in clinical trials.
Shift from modality-specific imaging to integrated multi-omics platforms
There is a growing transition from traditional single-modality imaging approaches (CT, MRI, PET) to integrated platforms that combine imaging biomarkers with genomic, proteomic, and metabolomic data. This shift reflects a broader move toward precision medicine, where imaging data is contextualized within molecular and biological frameworks to uncover deeper insights into disease mechanisms. The convergence of imaging and multi-omics analytics allows researchers to identify early predictive markers, improving patient stratification and therapeutic targeting in oncology and neurodegenerative diseases.
Market Summary
| Market Metric | Details & Data (2025-2034) |
|---|---|
| 2025 Market Valuation | USD 14.15 Billion |
| Estimated 2026 Value | USD 15.89 Billion |
| Projected 2034 Value | USD 40.13 Billion |
| CAGR (2026-2034) | 12.32% |
| Dominant Region | North America |
| Fastest Growing Region | Asia Pacific |
| Key Market Players | Alamar Biosciences, Inc., IXICO plc, Quibim, Siemens Healthineers AG, GE HealthCare |
to learn more about this report Download Free Sample Report
Market Driver
Rising focus on early therapeutic response monitoring in clinical research
Pharmaceutical and biotechnology companies are increasingly adopting imaging biomarkers to evaluate early treatment responses during drug development. By providing non-invasive, real-time insights into pharmacodynamic effects, imaging biomarkers reduce trial durations and improve go/no-go decision accuracy. This growing use in adaptive trial designs enhances clinical efficiency and accelerates the approval of targeted therapies across oncology, neurology, and cardiovascular research.
Market Restraint
Complex standardization and validation challenges across imaging sites
The variability in imaging equipment, acquisition parameters, and data processing workflows across research institutions poses a major challenge to biomarker reproducibility. Establishing standardized imaging protocols and harmonized analysis pipelines requires major coordination among stakeholders, limiting large-scale multi-center validation. These inconsistencies affect biomarker dependability and slow regulatory acceptance in drug trials and clinical diagnostics.
Market Opportunity
Growing application of AI-powered imaging biomarkers in mental health and neuropsychiatry
Emerging AI algorithms are being used to extract subtle imaging biomarkers from functional MRI and PET scans to study brain connectivity and neurochemical alterations associated with depression, anxiety, and schizophrenia. This evolution is opening a new frontier for imaging biomarkers beyond traditional oncology and neurology applications. The integration of imaging biomarkers in neuropsychiatric research could enable early detection, personalized therapy planning, and improved patient outcomes in mental health care, representing a rapidly expanding opportunity for market players.
Regional Analysis
North America dominated the imaging biomarkers market in 2025, accounting for 36.43% of the market share. This dominance is attributed to the region’s early integration of AI-driven imaging analytics and quantitative radiomics in clinical workflows, enabling high diagnostic precision across oncology and neurology applications.
The U.S. imaging biomarkers market continues to expand due to strong public-private collaborations between federal research institutions and pharmaceutical companies. These collaborations accelerate the translation of imaging biomarkers into clinical endpoints, particularly in Alzheimer’s disease and immuno-oncology trials. The U.S. is also seeing rising investment in cloud-based image repositories, enhancing the scalability of multi-center biomarker validation studies.
Asia Pacific Market Insights
The Asia Pacific region is growing with the fastest CAGR of 14.46 % from 2026 to 2034 in the imaging biomarkers market, supported by increasing investments in healthcare digitization and clinical imaging infrastructure. The expansion of pharmaceutical R&D pipelines in countries like China, India, and South Korea is encouraging the use of imaging biomarkers in drug development and translational research.
China Market China is emerging as a key regional contributor owing to government-backed initiatives that promote imaging data standardization and AI integration in diagnostic imaging. The availability of cost-efficient imaging systems and a large clinical trial population positions China as a leading hub for imaging biomarker validation studies.
Regional Market share (%) in 2025
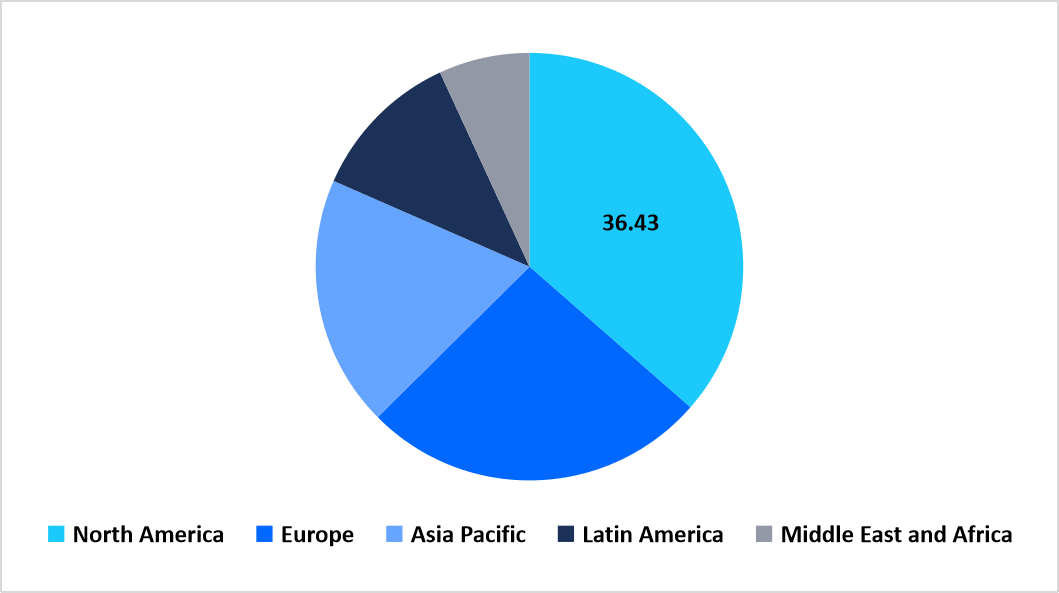
Source: Straits Research Analysis
Europe Market Insights
Europe is projected to grow steadily in the imaging biomarkers market, driven by cross-border research initiatives and harmonized imaging standards supported by the European Union. The region’s emphasis on precision medicine and data sharing is fostering advancements in imaging biomarker quantification and clinical trial reproducibility.
Germany Market: Germany is witnessing growing adoption of imaging biomarkers due to its expanding network of hybrid PET/MRI facilities that integrate molecular and anatomical data for early disease detection. Academic institutions and imaging centers are forming alliances to establish reference biomarker datasets, supporting regulatory submissions and clinical decision-making.
Latin America Market Insights
Latin America’s imaging biomarkers market is developing as healthcare systems modernize and prioritize advanced diagnostic imaging capabilities. Growing partnerships between radiology networks and academic research institutions are enhancing the use of imaging biomarkers for early cancer detection and neurodegenerative disease monitoring.
Brazil Market: Brazil’s market growth is driven by increasing access to advanced PET/CT and MRI imaging facilities in urban centers. The establishment of national oncology screening programs is expanding the utilization of quantitative imaging biomarkers for evaluating tumor progression and therapy response, promoting data-driven clinical outcomes.
Middle East and Africa Market Insights
The Middle East and Africa region is expanding due to substantial investments in healthcare infrastructure and diagnostic imaging modernization projects. Countries in the Gulf Cooperation Council (GCC) are focusing on integrating imaging biomarkers into tertiary hospitals to enhance precision diagnostics and patient monitoring.
United Arab Emirates Market: In the UAE, the rising prevalence of cardiovascular and metabolic disorders is encouraging hospitals to incorporate imaging biomarkers into routine diagnostics for early risk assessment. The adoption of advanced imaging modalities like cardiac MRI and CT angiography is creating new opportunities for quantitative biomarker applications, strengthening the country’s focus on outcome-based healthcare.
Type Insights
The molecular/nuclear biomarkers segment held the largest market share of 36.63% in 2025, due to the rising adoption of radiotracers and molecular probes that enable visualization of cellular and metabolic activity at the molecular level.
The quantitative imaging biomarkers (QIB) segment is anticipated to register a CAGR of 13.78% over the forecast period, owing to the increasing demand for objective and reproducible imaging measurements that enhance diagnostic accuracy and clinical trial efficiency.
Technology Insights
The computed tomography (CT) segment dominated the market in 2025 with a 32.34% share. CT-based imaging biomarkers are widely utilized for their high spatial resolution, allowing detailed quantification of tumor burden, tissue density, and lesion morphology across oncology and pulmonary research. The segment benefits from extensive use in longitudinal imaging studies and regulatory-approved imaging protocols that rely on standardized CT parameters for disease progression assessment.
The positron emission tomography (PET) segment is projected to grow at the fastest rate of 13.13%. The growth is driven by its ability to visualize metabolic activity and molecular processes in vivo, supporting early-stage drug trials and precision oncology. Increasing adoption of radiotracers such as FDG and novel ligands for neuroimaging and immunotherapy response evaluation is accelerating PET’s market penetration.
Application Insights
The diagnostics segment dominated the imaging biomarkers market in 2025, with a 37.38% share. Its dominance is due to the extensive use of imaging biomarkers in early disease detection and clinical evaluation, particularly in neurological and cardiovascular disorders. The segment’s expansion is supported by hospital-based diagnostic imaging centers that utilize standardized biomarker quantification methods to improve diagnostic accuracy and patient outcomes.
The drug discovery and development segment is expected to register the fastest growth of 13.68%. The integration of imaging biomarkers in clinical trial design enables visualization of pharmacodynamic responses and real-time assessment of therapeutic efficacy. Pharmaceutical companies increasingly rely on imaging biomarkers to accelerate go/no-go decisions in early-phase trials and reduce development timelines for novel therapeutics.
End User Insights
The hospitals segment held the largest share of 41.27% in 2025. Hospitals serve as key hubs for clinical imaging research and patient management, leveraging advanced modalities such as PET/CT and MRI to guide treatment planning and monitor therapy response. The segment’s growth is further supported by multi-specialty collaborations between radiology and oncology departments, promoting imaging biomarker utilization in integrated care pathways.
The diagnostic and imaging centers segment is anticipated to expand at the fastest CAGR of 13.32% during the forecast period. These centers are increasingly investing in high-throughput imaging systems and data analytics tools to support biomarker-based image interpretation. The availability of cloud-enabled platforms for image sharing and automated quantification is enabling independent imaging facilities to participate in multicentric biomarker studies and broaden their research capabilities.
By End User Market Share (%), 2025
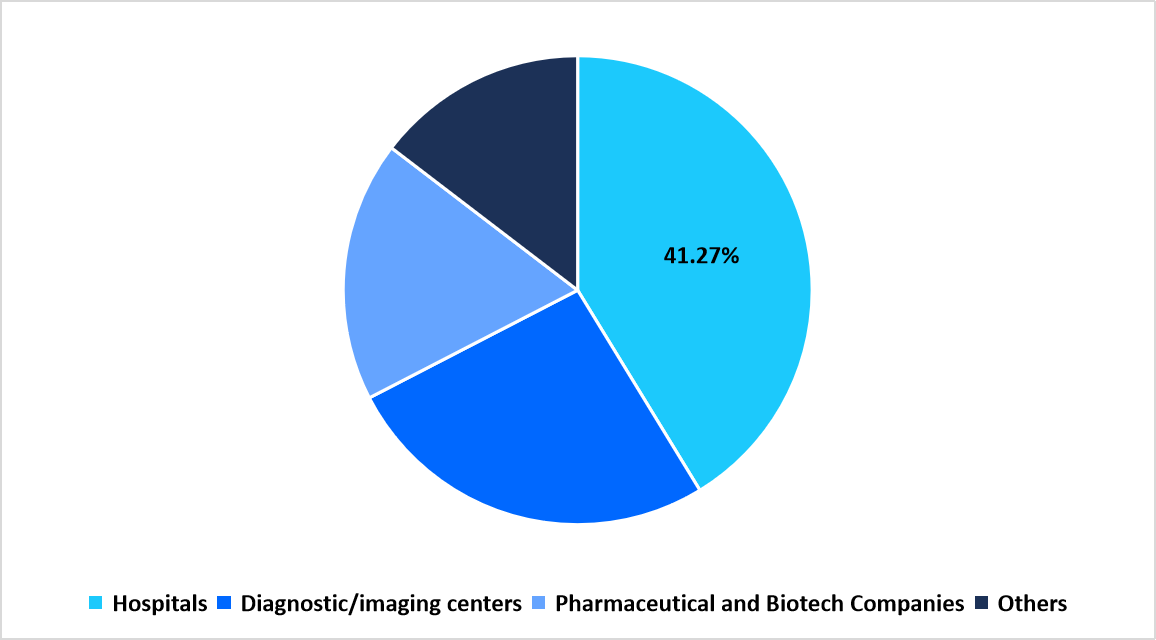
Source: Straits Research
Competitive Landscape
The global imaging biomarkers market is moderately fragmented, featuring a mix of established medical imaging companies, biotechnology firms, and AI-driven diagnostic solution providers.
Alamar Biosciences, Inc.: An emerging market player
Alamar Biosciences has emerged as a notable innovator in the imaging biomarkers landscape, focusing on ultrasensitive protein detection technologies.
In October 2025, Alamar Biosciences, Inc., introduced the NULISAqpcr BD-pTau217 Assay, marking a major advancement in non-invasive, brain-specific biomarker detection for Alzheimer’s disease research. The assay enabled precise measurement of brain-derived phosphorylated tau 217 (pTau217) in blood or serum using only 10 µL of sample, eliminating the demand for cerebrospinal fluid collection or PET imaging and facilitating large-scale clinical and population-based studies.
List of Key and Emerging Players in Imaging Biomarkers Market
- Alamar Biosciences, Inc.
- IXICO plc
- Quibim
- Siemens Healthineers AG
- GE HealthCare
- Koninklijke Philips NV
- CANON MEDICAL SYSTEMS CORPORATION
- FUJIFILM Holdings Corporation
- Bruker
- AGFA HealthCare
- Hologic, Inc.
- Revvity Inc.
- Clario
- Merck KGaA
- Thermo Fisher Scientific Inc.
- Median Technologies
- Bio-Rad Laboratories, Inc.
- Abcam Limited
- QIAGEN
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- Shimadzu Corporation
- Konica Minolta, Inc.
- Others
Strategic Initiatives
- June 2025: IXICO plc, based in the UK, collaborated with Fujirebio Diagnostics to secure FDA clearance for the Lumipulse G pTau 217/β-Amyloid 1-42 Plasma Ratio test, the first blood-based diagnostic biomarker for Alzheimer’s disease. Utilizing IXICO’s AI-powered imaging analytics platform, the validation incorporated data from the Bio-Hermes-001 study conducted with the Global Alzheimer’s Platform Foundation.
- February 2024: Quibim unveiled QP-Insights, an integrated web-based platform designed for secure management, storage, and quantitative analysis of medical images and related clinical data. The platform supported the deployment of imaging-based algorithms to accelerate drug development and optimize clinical trial workflows.
Report Scope
| Report Metric | Details |
|---|---|
| Market Size in 2025 | USD 14.15 Billion |
| Market Size in 2026 | USD 15.89 Billion |
| Market Size in 2034 | USD 40.13 Billion |
| CAGR | 12.32% (2026-2034) |
| Base Year for Estimation | 2025 |
| Historical Data | 2022-2024 |
| Forecast Period | 2026-2034 |
| Report Coverage | Revenue Forecast, Competitive Landscape, Growth Factors, Environment & Regulatory Landscape and Trends |
| Segments Covered | By Type, By Technology, By Application, By End User |
| Geographies Covered | North America, Europe, APAC, Middle East and Africa, LATAM |
| Countries Covered | US, Canada, UK, Germany, France, Spain, Italy, Russia, Nordic, Benelux, China, Korea, Japan, India, Australia, Taiwan, South East Asia, UAE, Turkey, Saudi Arabia, South Africa, Egypt, Nigeria, Brazil, Mexico, Argentina, Chile, Colombia |
to learn more about this report Download Free Sample Report
Imaging Biomarkers Market Segments
By Type
- Anatomical biomarkers
- Functional biomarkers
- Molecular/Nuclear Biomarkers
- Quantitative Imaging Biomarkers
- Others
By Technology
- Positron Emission Tomography (PET)
- Ultrasound, Magnetic Resonance Imaging
- Computed Tomography (CT)
- Single-Photon Emission Computerized Tomography (SPECT)
- Others
By Application
- Diagnostics
- Drug Discovery & Development
- Personalized Medicine
- Disease Risk Assessment
- Others
By End User
- Hospitals
- Diagnostic/imaging centers
- Pharmaceutical and Biotech Companies
- Others
By Region
- North America
- Europe
- APAC
- Middle East and Africa
- LATAM
Frequently Asked Questions (FAQs)
Debashree Bora
Healthcare Lead
Debashree Bora is a Healthcare Lead with over 7 years of industry experience, specializing in Healthcare IT. She provides comprehensive market insights on digital health, electronic medical records, telehealth, and healthcare analytics. Debashree’s research supports organizations in adopting technology-driven healthcare solutions, improving patient care, and achieving operational efficiency in a rapidly transforming healthcare ecosystem.










































